Method for preparing N-substituted-L-methyl tryptophan ester
A technology of tryptophan methyl ester and tryptophan, which is applied in the field of preparation of N-substituted L-tryptophan methyl ester, can solve the problems of long reaction time, unfavorable actual production, high cost, etc., and achieve shortened reaction time and high production efficiency. Effects of cost reduction and utilization efficiency improvement
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The preparation method of N-substituted L-tryptophan methyl ester of the present invention comprises the following steps:
[0030] 1) Starting from L-tryptophan 1, methylate L-tryptophan methyl ester hydrochloride 2;
[0031] 2) Dehydrochlorination of L-tryptophan methyl ester hydrochloride under alkaline conditions to obtain L-tryptophan methyl ester;
[0032] 3) Condensation reaction of L-tryptophan methyl ester and aromatic aldehyde to obtain imine 3;
[0033] 4) Using sodium borohydride as a reducing agent, the imine is selectively reduced to N-substituted L-tryptophan methyl ester 4.
Embodiment 1
[0036] Step 1: Preparation of L-tryptophan methyl ester hydrochloride:
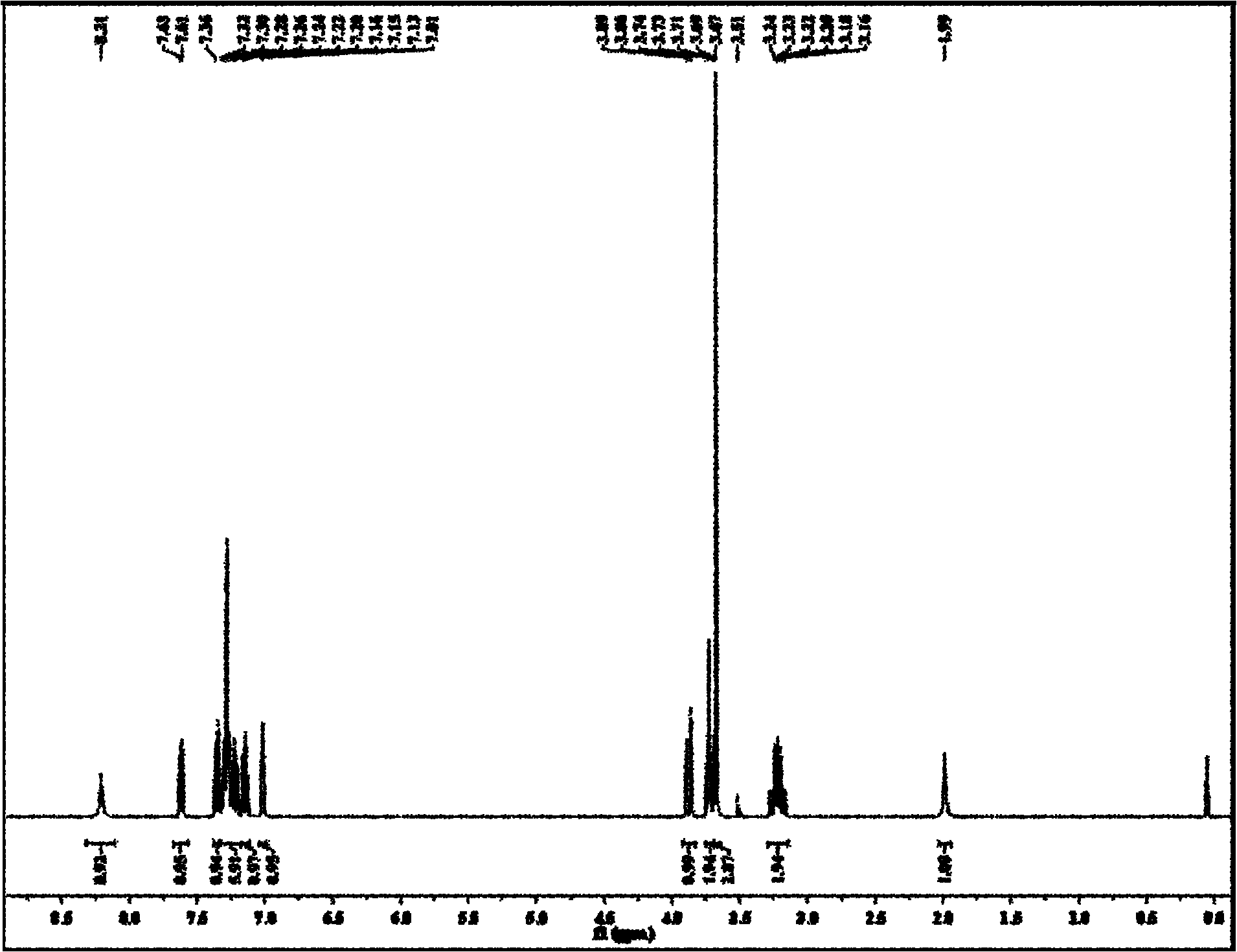
[0037] Add 50ml of anhydrous methanol to a 100ml three-neck flask, keep the temperature below 0°C in an ice-salt bath, turn on the stirrer, and after the methanol is completely cooled, slowly add 3.27ml of freshly distilled thionyl chloride dropwise with a dropping funnel (45mmol), keep the flow rate as 15 drops per minute, continue to react for 30min after dripping, slowly rise to room temperature and continue to stir for 2h to form solution A; get 6.12g (30mmol) L-tryptophan into solution A, heat up Reflux, and continue to reflux for 5 hours after the L-tryptophan dissolves completely. After the reaction is completed, the solvent methanol and residual thionyl chloride are distilled off under reduced pressure to obtain a white solid, which is finally recrystallized in a mixed solution of methanol and ether. The volume ratio of methanol to diethyl ether was 5:1 to obtain L-tryptophan methyl ester hydrochl...
Embodiment 2
[0051] The step 1, i.e. the preparation method of L-tryptophan methyl ester hydrochloride is the same as in Example 1, so only step 2 is described here, specifically as follows:
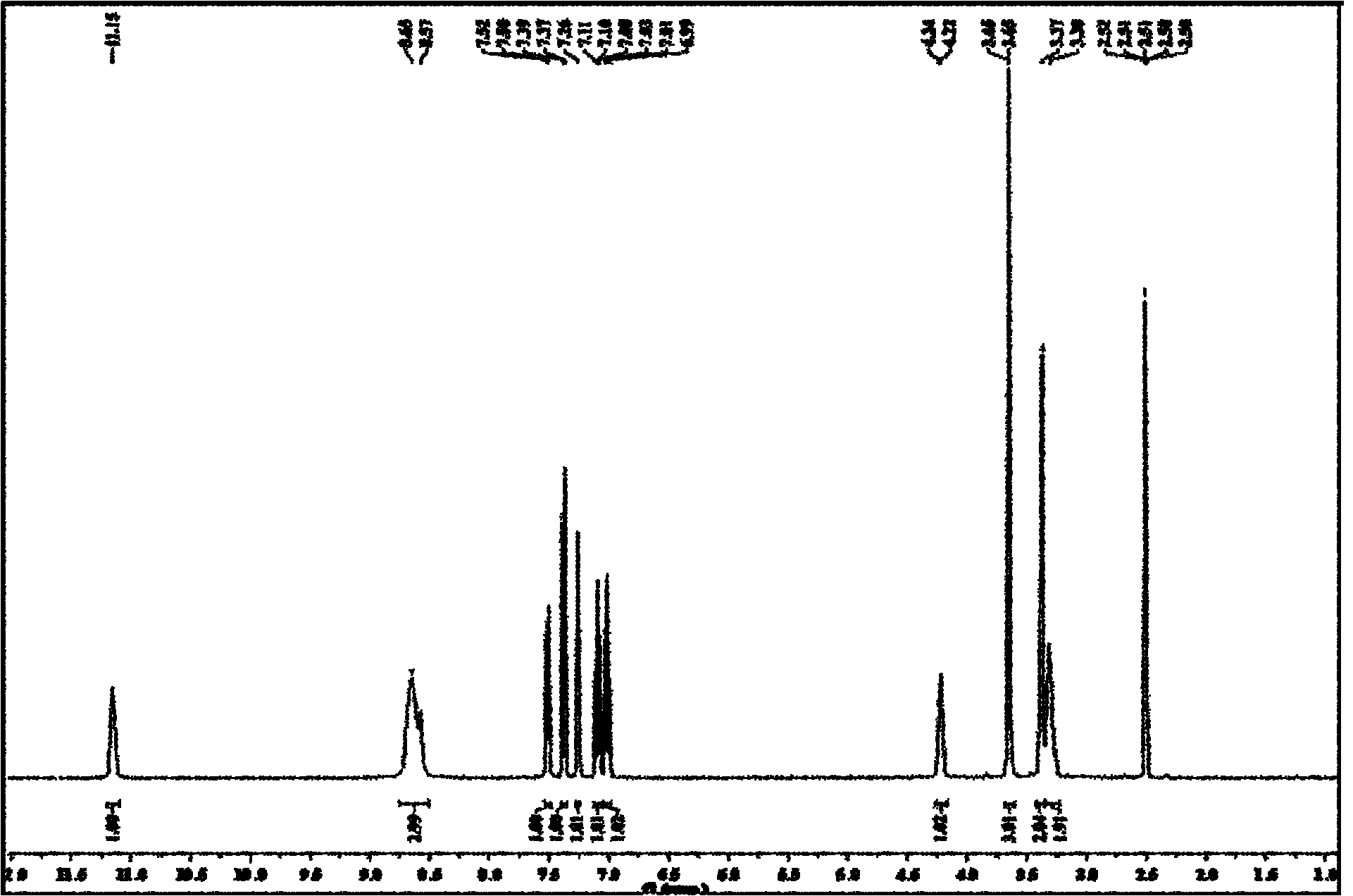
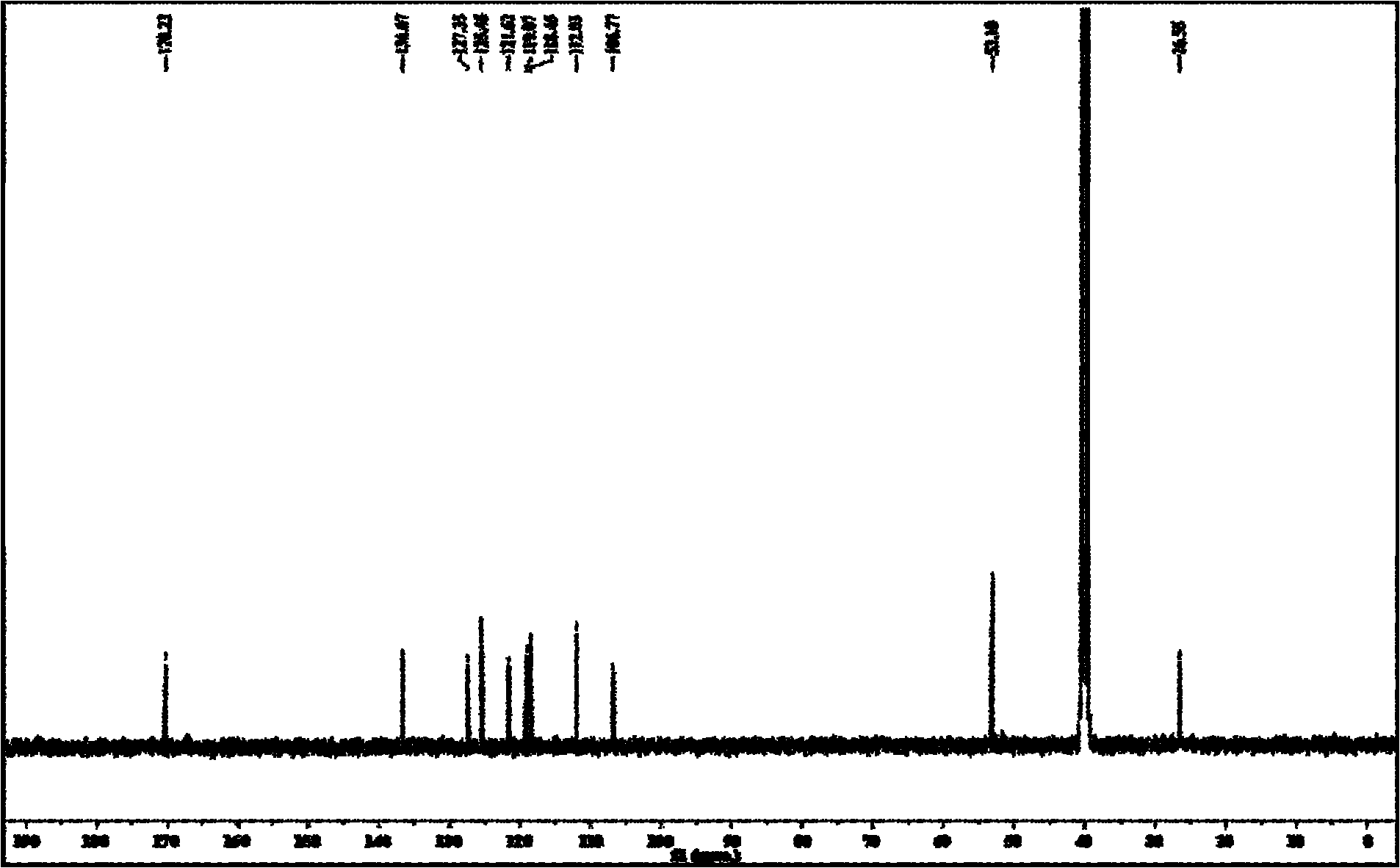
[0052] Take 2.03g (8mmol) of L-tryptophan methyl ester hydrochloride and add it to a 100ml three-necked flask, add 20ml of anhydrous methanol and stir until dissolved, add 0.8g (8mmol) of triethylamine under ice-salt bath conditions and continue After stirring for 1 h, a colorless solution was obtained, and then N 2 Drop 8 mmol of anisaldehyde under protection, react below 0°C for 3 hours, then add sodium borohydride in batches, the total amount of sodium borohydride added is 0.363 g (9.6 mmol), continue stirring for 1 hour, and then add water to decompose excess borohydride Sodium and dichloromethane extracted, the organic phase was evaporated to dryness, and the residue was recrystallized with a mixed solvent of chloroform and methanol in an appropriate proportion to obtain the target compound as c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com