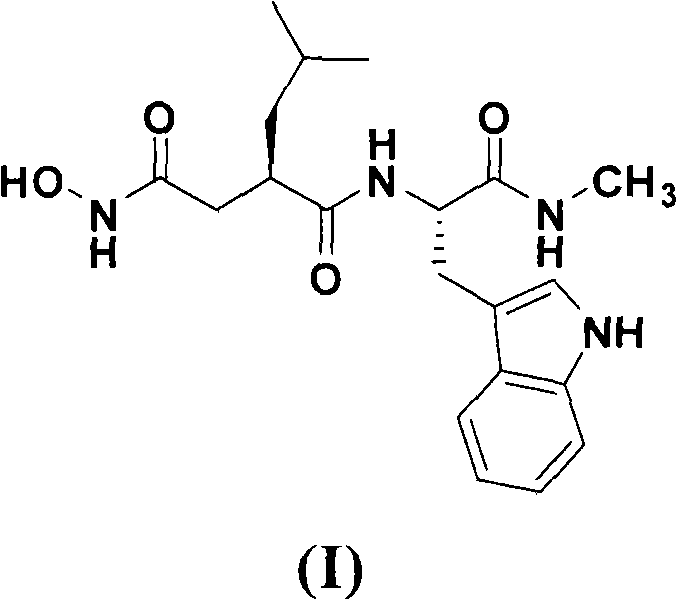
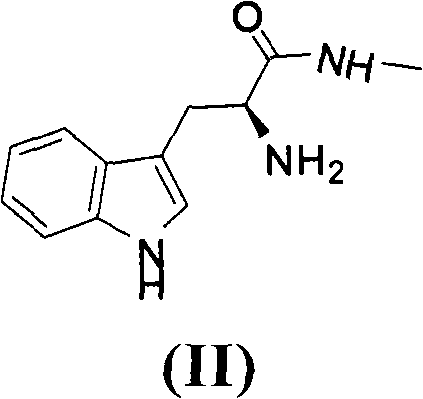
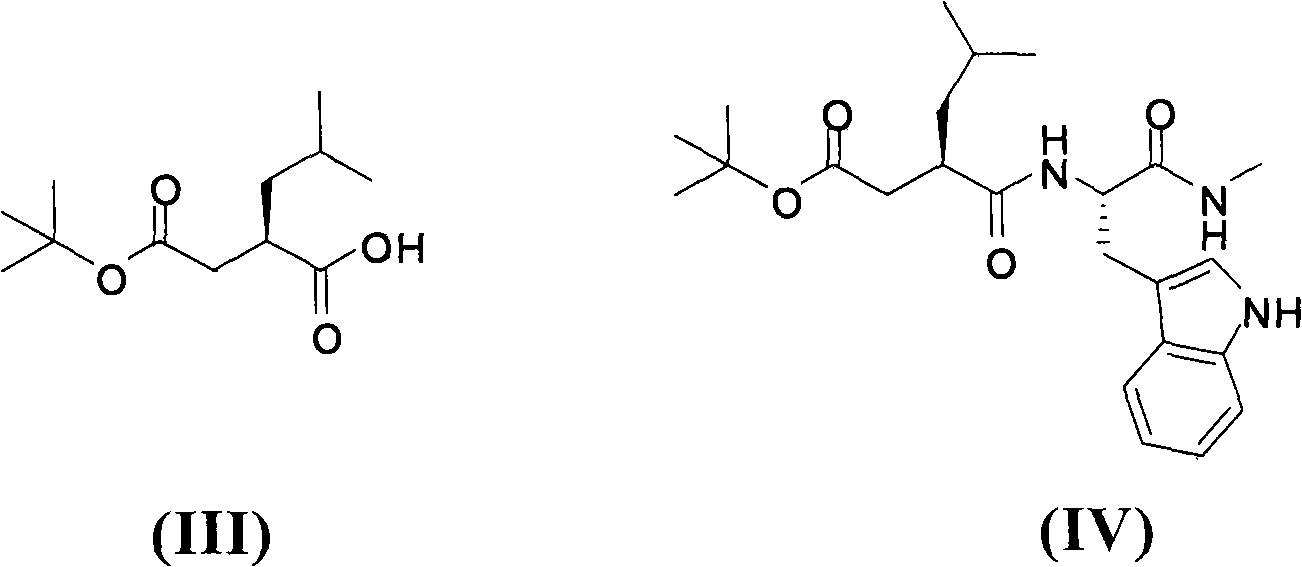
Method for preparing ilomastat
A technology for ilomastat and intermediates, which is applied in the field of preparation of ilomastat, can solve the problems of cumbersome operation and dark product color, and achieve the effects of increasing solubility, high condensation efficiency, and not easy to deliquescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0044] The present invention will be further illustrated by specific preparation examples below, however, it should be understood that these examples are only used for more detailed description and should not be construed as limiting the present invention in any form. In the description of the present invention, unless otherwise specified, the reagents, raw materials, intermediates, solvents, etc. used can be obtained from commercial sources or prepared by methods known to those skilled in the art.
[0045] 1, Preparation of L-Tryptophanamide (II)
[0046] Mix L-tryptophan methyl ester hydrochloride (144.74g, 0.57mol) with methylamine aqueous solution (25-30%) 430mL, stir at room temperature for 7 hours, use dichloromethane to carry out continuous extraction for 5 hours, separate , the obtained organic phase was dried with anhydrous magnesium sulfate, and the solvent was evaporated under reduced pressure to obtain a light yellow solid, which was washed with P 2 o 5 After dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com