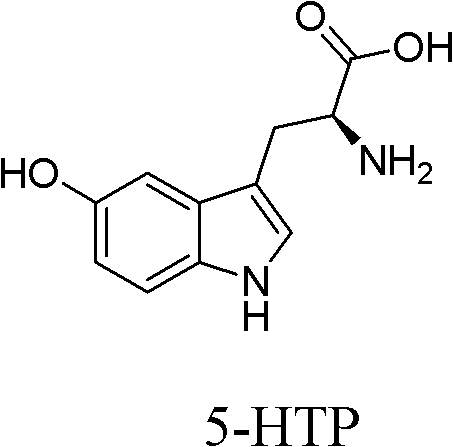
Preparation method of levo-5-hydroxytryptophan
A technology of hydroxytryptophan and tryptophan methyl ester, which is applied in organic chemistry and other fields, and can solve the problem that the plant extraction process is affected by seasons, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
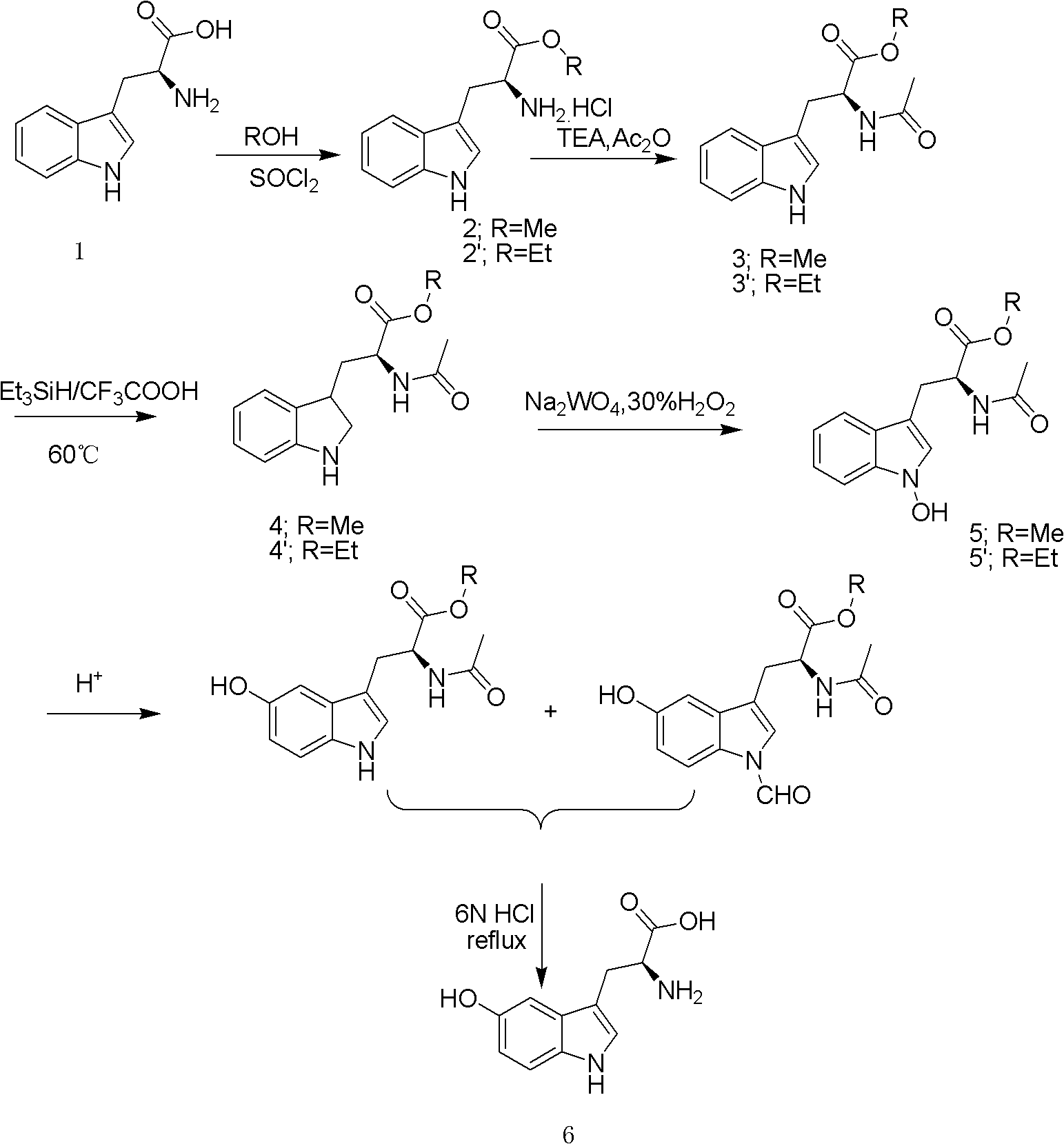
[0036] The preparation method of L-5-hydroxytryptophan of the present invention comprises the following steps:
[0037] 1) Starting from L-tryptophan (1), esterify methyl / ethyl to obtain L-tryptophan methyl / ethyl hydrochloride (2);
[0038] 2) Dehydrochloride L-tryptophan methyl ester / ethyl ester hydrochloride under alkaline conditions to obtain L-tryptophan methyl ester / ethyl ester, and then acetylate it into N-acetyl-L-tryptophan methyl ester / ethyl ester (3);
[0039] 3) Reducing the indole ring with N-acetyl-L-tryptophan methyl / ethyl ester in a triethylsilane-trifluoroacetic acid reduction system to obtain compound (4);
[0040] 4) Compound (4) was oxidized in the 1-position nitrogen of the indole ring under sodium tungstate-30% hydrogen peroxide system to obtain compound (5);
[0041] 5) Compound (5) transfers the hydroxyl group on the 1-position nitrogen of the indole ring to the 5-position carbon of the benzene ring under acidic conditions, and deacetylates to obtain ...
Embodiment 1
[0043] Step 1: Preparation of L-tryptophan methyl ester hydrochloride (2):
[0044] Weigh 174g of L-tryptophan (1) in 1.6L of methanol, cool to 0°C, add 87mL of thionyl chloride dropwise at 0°C, after the dropwise addition, raise the temperature to 30°C and react for 6 hours. After the reaction was completed, the solvent was distilled off under reduced pressure to obtain a white solid. The solid was washed with 300 mL of ether to obtain L-tryptophan methyl ester hydrochloride, which weighed 214 g.
[0045] The characterization parameters of this L-tryptophan methyl ester hydrochloride are as follows:
[0046] Melting point: 232~233℃;
[0047] Mass spectral data: C 12 h 15 ClN 2 o 2 ,[M+H] + = 255.1;
[0048] NMR data:1 H NMR (δ, ppm, DMSO-d6, 400MHz): 3.39 (2H, m, CH 2 ); 3.63 (3H, s, CH 3 O); 4.20 (1H, t, CH, J = 5.5Hz); 7.07 (2H, dt, J = 21Hz, 6Hz); 7.26 (1H, d, J = 3Hz); 7.39 (1H, d, J = 7.8 Hz).
[0049] Step 2: Preparation of N-acetyl-L-tryptophan methyl ester...
Embodiment 2
[0076] Step 1: Preparation of L-tryptophan ethyl ester hydrochloride (2):
[0077] Weigh 174g of L-tryptophan (1) in 1.6L of ethanol, cool to 0°C, add 140mL of thionyl chloride dropwise at 2°C, after the dropwise addition, raise the temperature to 45°C and react for 4 hours. After the reaction was completed, the solvent was distilled off under reduced pressure to obtain a white solid. The solid was washed with 200 mL of diethyl ether to obtain L-tryptophan ethyl ester hydrochloride, which weighed 217.5 g.
[0078] The characterization parameters of this L-tryptophan ethyl ester hydrochloride are as follows:
[0079] Mass spectral data: C 13 h 17 ClN 2 o 2 ,[M+H] + = 269.1;
[0080] NMR data: 1 H NMR (δ, ppm, DMSO-d6, 400MHz): 1.30 (3H, t, CH 3 ); 3.39 (2H, m, CH 2 ); 4.23 (2H, CH 3 O); 4.20 (1H, t, CH, J = 5.5Hz); 7.07 (2H, dt, J = 21Hz, 6Hz); 7.26 (1H, d, J = 3Hz); 7.39 (1H, d, J = 7.8 Hz).
[0081] Step 2: Preparation of N-acetyl-L-tryptophan ethyl ester (3):
...
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com