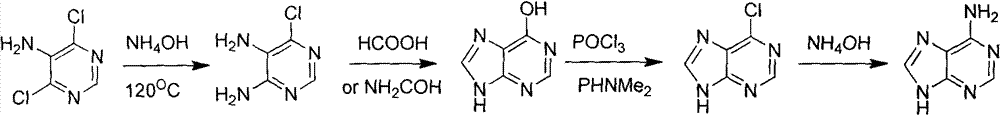
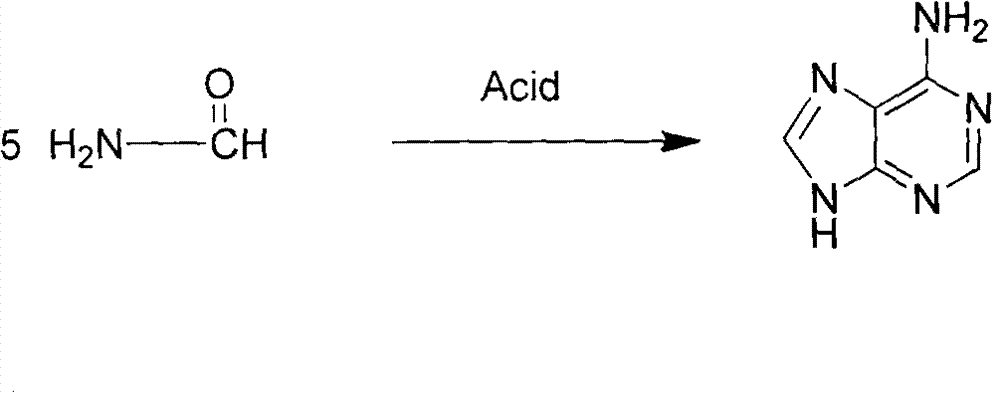
Novel chemical synthesis method for adenine
A chemical synthesis, adenine technology, applied in the field of chemical synthesis of adenine, can solve the problems of many side reactions, difficult to obtain pure products, cumbersome post-processing, etc., achieve low production cost, shorten the route steps, and improve production efficiency.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
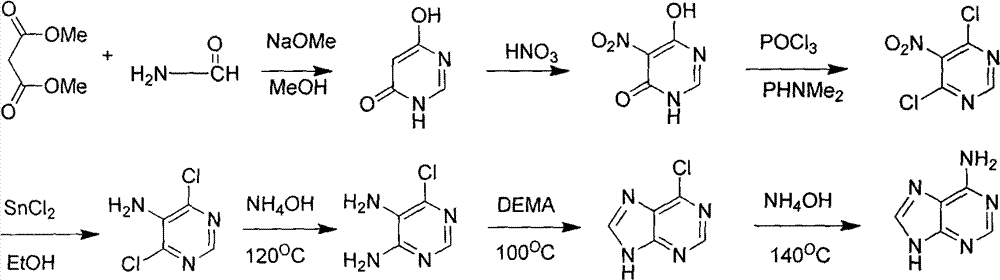
[0051] Embodiment 1: the synthesis of raw material 4,6-dihydroxypyrimidine
[0052] Add 2500 mL of absolute ethanol to a 5000 mL three-necked round bottom flask equipped with stirring, and then slowly add 150 g of metallic sodium until the metallic sodium basically disappears to obtain a transparent solution. Begin to heat up slowly. Measure 200 mL of formamide, and slowly drop it into the sodium ethoxide solution with a constant pressure dropping funnel. At 65-70° C., continue to slowly add 310 mL of diethyl malonate dropwise after the drop, and the solution starts to turn white. After dropping, adjust the temperature to 110° C., and continue heating and stirring overnight. After the solution was cooled to room temperature, most of the ethanol was distilled off under reduced pressure. Then, the remaining residue was treated with a mixed solution of 450 mL of concentrated hydrochloric acid and 750 mL of ice water at 0°C. Suction filter, wash with ice water, and dry under r...
Embodiment 2
[0053] Example 2: Synthesis of 4,6-dihydroxy-5-nitropyrimidine
[0054]Pour 500mL of concentrated nitric acid and 100mL of concentrated sulfuric acid into a 2500mL three-neck flask equipped with stirring. Weigh 112g of 4,6-dihydroxypyrimidine, and add it to the mixed acid solution in batches under ice-bath conditions, and keep stirring. The solution turned dark red. After the addition, the reaction was continued at 0°C for 90 minutes, and then continued at room temperature for 3 hours. The reaction solution was poured into 5000 mL of crushed ice, solids were precipitated, filtered, washed with water, recrystallized from absolute ethanol, and dried in vacuo. 110 g of solid were obtained. The yield is about 70%.
Embodiment 3
[0055] Example 3: Synthesis of 4,6-dichloro-5-nitropyrimidine
[0056] Add 500mL POCI to the three-necked flask 3 , and then slowly added 80g of 4,6-dihydroxy-5-nitropyrimidine, heated to 50°C and added 100mL of N,N-dimethylaniline, heated to reflux for 2h. After the solution was cooled to room temperature, excess phosphorus oxychloride was recovered by distillation under reduced pressure. After concentration, the dark black solution obtained was poured into 5000 mL of ice water and extracted with ethyl acetate (3×500 mL). The organic layers were combined and dried over anhydrous magnesium sulfate. The ethyl acetate solution was concentrated under reduced pressure to obtain a black solid, which was recrystallized from petroleum ether at 60-90°C to obtain 75 g of yellow crystals, with a yield of about 76%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com