Preparation method for (R)-4-hydroxy-2-oxo-1-pyrrolidine acetamide
A pyrrolidine acetamide, -4- technology, applied in the direction of organic chemistry, etc., can solve the problems of increased impurity content, unfavorable industrial production, high enantiomer content, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
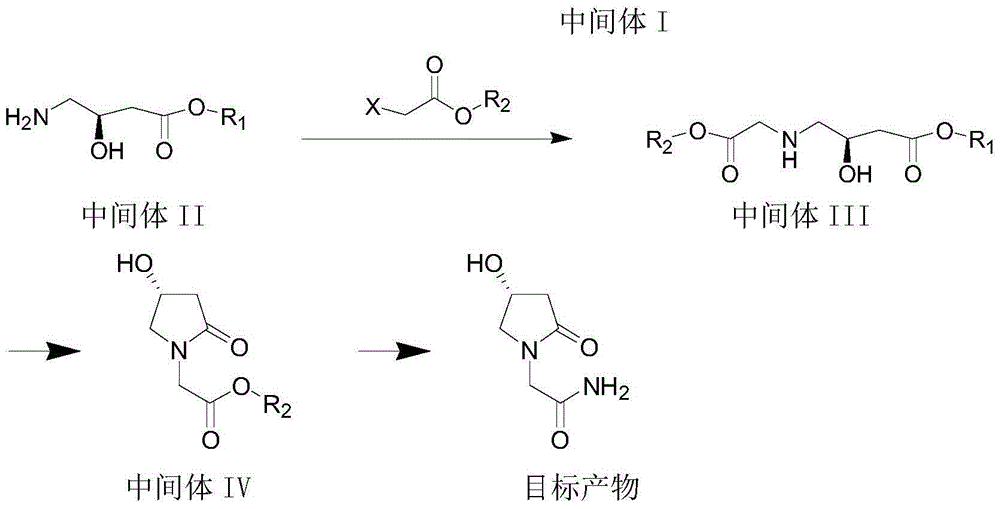
[0066] A kind of synthetic method of (R)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide, it is carried out as follows,
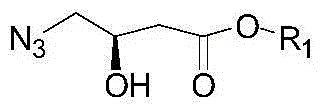
[0067] (1) Preparation of Intermediate I:
[0068] Take 50g of the raw material R-methyl 4-chloro-3-hydroxybutyrate, put it into a single-necked bottle, add 50ml of DMF, stir, cool in an ice-water bath, add 50g of sodium azide, keep the temperature not exceeding 40°C, after the addition The temperature was raised to 60°C. After 2 hours of reaction, the reaction was stopped to obtain a yellow solution. Add 100 ml of water, extract with 100 ml of ethyl acetate, concentrate to remove ethyl acetate, and obtain Intermediate I as a yellow oil. After nuclear magnetic detection, the intermediate I is: 1H-NMR (300MHz, CDCl3): δ1.42-1.73 (m, 2H) 2.76-2.67 (ABsystem, m, 2H,), 3.31-3.23 (ABsystem, m, 2H) , 3.75(s, 3H), 4.40(m, 1H), 3.70(s, 1H). Intermediate I is: R1 is methyl.
[0069] (2) Preparation of Intermediate II
[0070] The intermediate I obtained in step (2) w...
Embodiment 2
[0080] A kind of synthetic method of (R)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide, it is carried out as follows,
[0081] (1) Preparation of Intermediate I:
[0082]Weigh 50kg of methyl R-4-chloro-3-hydroxybutyrate, add it to a 500L azidation reaction kettle, add 50L of DMF, stir evenly, cool the jacket in an ice-water bath, add 50Kg of sodium azide, and keep the temperature constant When the temperature exceeds 40°C, the ice water in the jacket is pressed out, and the jacket is passed through hot water to raise the internal temperature to 60°C. After 2 hours of reaction, the reaction was stopped to obtain a yellow solution. Add 100 L of water to the kettle, extract with 100 L of ethyl acetate, separate and discard the water phase, concentrate the organic phase to remove ethyl acetate, and obtain Intermediate I as a yellow oil. After nuclear magnetic detection, the intermediate I is: 1H-NMR (300MHz, CDCl3): δ1.42-1.73 (m, 2H) 2.76-2.67 (ABsystem, m, 2H,), 3.31-3.23 (ABsystem,...
Embodiment 3
[0094] 1, a kind of synthetic method of (R)-4-hydroxyl-2-oxo-1-pyrrolidineacetamide, as follows:
[0095] (1) Stir R-4-chloro-3-hydroxybutyric acid ethyl ester with 18 times the weight of DMSO and 1 times the weight of sodium azide, and react with azidation at 60°C for about 5 hours. The raw materials are basically The reaction is complete, stop the reaction, directly concentrate to remove the solvent, and solidify at low temperature to obtain intermediate I; meanwhile, the above solvents also use DMF, n-propanol, isopropanol, n-butanol, tert-butanol, toluene or cyclopentanol, etc. Preparation of intermediate I, and finally by nuclear magnetic detection, the prepared intermediate I is: 1H-NMR (300MHz, CDCl3): δ1.42-1.73 (m, 5H) 2.76-2.67 (ABsystem, m, 2H,), 3.31-3.23 (ABsystem, m, 2H), 4.40 (m, 1H), 3.70 (s, 1H).
[0096] (2) With the intermediate I obtained from step (1), in 15 weight times of ethanol of R-4-chloro-3-hydroxybutyrate ethyl ester, add 0.5 times of weight of 10...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com