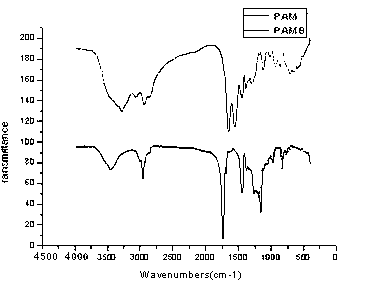
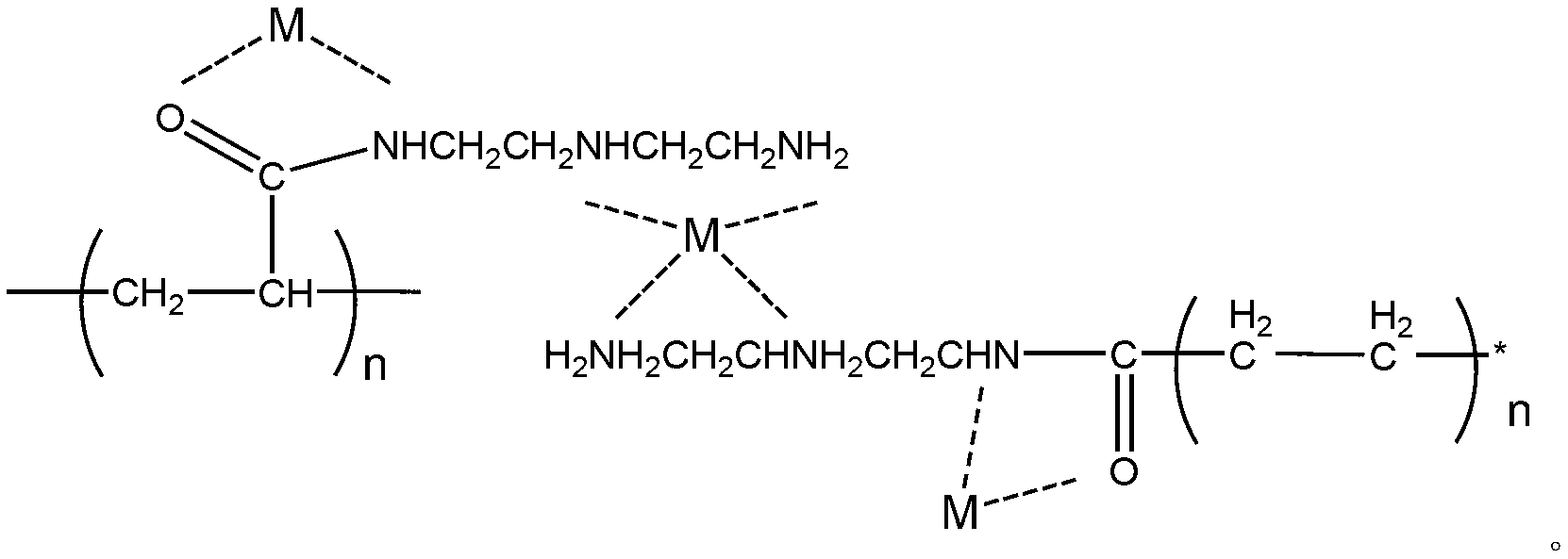
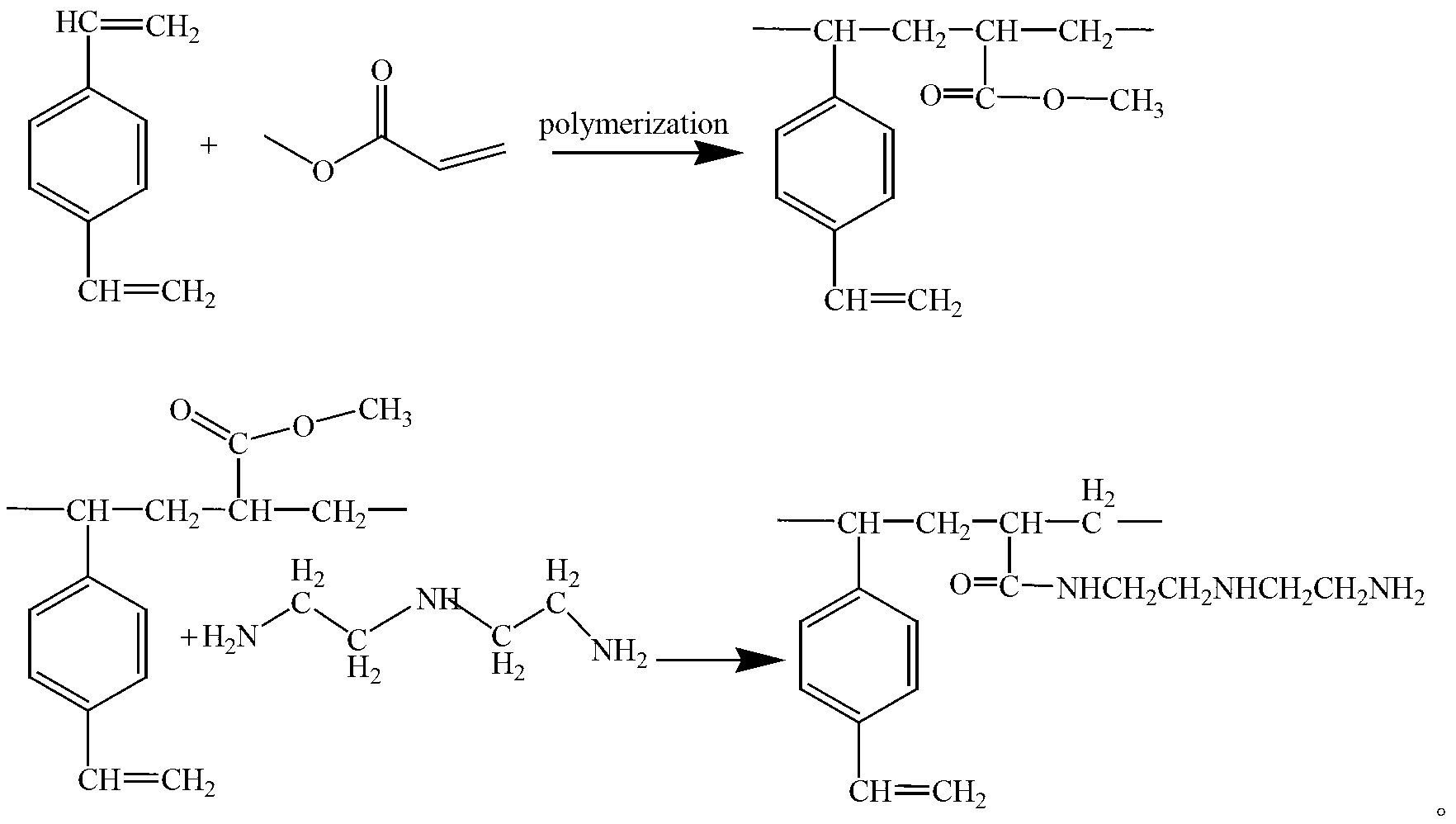
Acrylic-acid high-capacity primary-amino chelate resin for trapping copper ions and preparation method thereof
A chelating resin, high-capacity technology, used in chemical instruments and methods, other chemical processes, water/sludge/sewage treatment, etc., can solve the problems of complex synthesis paths and small adsorption capacity, and achieve simple operation steps and adsorption. The effect of large capacity and easy control of synthesis conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0047] The preparation method of the acrylic acid-based large-capacity primary amine-based chelating resin for capturing copper ions of the present example 1 includes the following steps:
[0048] 1) In a 500mL three-necked flask equipped with a stirrer, a reflux condenser and a thermometer, add 250mL of an aqueous solution containing 1% polyvinyl alcohol and 8% NaCl, at a stirring speed of 50rpm, heat up to 40°C and keep it for 1h.
[0049] 2) Add 25g of methyl acrylate, 1g of triallyl cyanurate, 15mL of ethyl acetate, 15mL of n-heptane, and 0.2g of benzoyl peroxide under a stirring speed of 50rpm and mix, and heat up at a rate of 5℃ / 15min Polymerize for 1 hour at 65°C, then heat up to 75°C, polymerize for 1 hour, continue to heat up to 85°C, polymerize for 1 hour, then heat up to 95°C, react at a constant temperature for 2 hours, and stop the reaction.
[0050] 3) The product was washed with hot water, cold water and ethanol, and dried in vacuum.
[0051] 4) Extract the porogen with...
Embodiment 2
[0057] The preparation method of the acrylic acid-based large-capacity primary amine-based chelating resin for capturing copper ions in Example 2 includes the following steps:
[0058] 1) Add 250 mL of an aqueous solution containing 3% gelatin and 23% NaCl to a 500 mL three-necked flask equipped with a stirrer, reflux condenser and thermometer. Under a stirring speed of 80 rpm, increase the temperature to 40°C and keep it for 1 hour.
[0059] 2) Add 25g of methyl methacrylate, 5g of triallyl cyanurate, 15mL of toluene, 30mL of n-heptane, 0.2g of benzoyl peroxide at a stirring speed of 80rpm and mix, and heat up at a rate of 5℃ / 15min Polymerize for 2 hours at 65°C, then heat up to 75°C, polymerize for 2 hours, continue to heat up to 85°C, polymerize for 2 hours, then heat up to 95°C, react at a constant temperature for 4 hours, and stop the reaction.
[0060] 3) The product was washed with hot water, cold water and ethanol, and dried in vacuum.
[0061] 4) Extract the porogen with meth...
Embodiment 3
[0067] The preparation method of the acrylic acid-based large-capacity primary amino chelating resin for capturing copper ions of Example 3 includes the following steps:
[0068] 1) In a 500mL three-necked flask equipped with a stirrer, a reflux condenser and a thermometer, add 250mL of an aqueous solution containing 1.5% Gul gum and 20% NaCl, and at a stirring speed of 100rpm, the temperature is raised to 40°C and kept for 1h.
[0069] 2) Add 25g ethyl acrylate, 1g trimethacrylic acid (trimethylolpropyl) ester, 15mL ethyl acetate, 40mL n-heptane, 0.2g benzoyl peroxide and mix at a stirring speed of 100rpm. At a rate of 30 min, the temperature is raised to 65°C, polymerization is performed for 5 hours, then the temperature is increased to 75°C, the polymerization reaction is 2 hours, the temperature is continued to rise to 85°C, the polymerization is 5 hours, and then the temperature is raised to 95°C, and the reaction is kept constant for 10 hours to stop the reaction.
[0070] 3) T...
PUM
| Property | Measurement | Unit |
|---|---|---|
| adsorption capacity | aaaaa | aaaaa |
| adsorption capacity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com