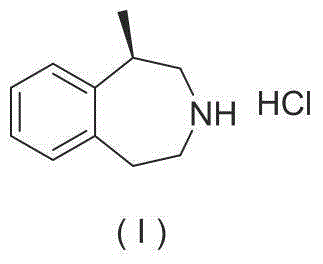
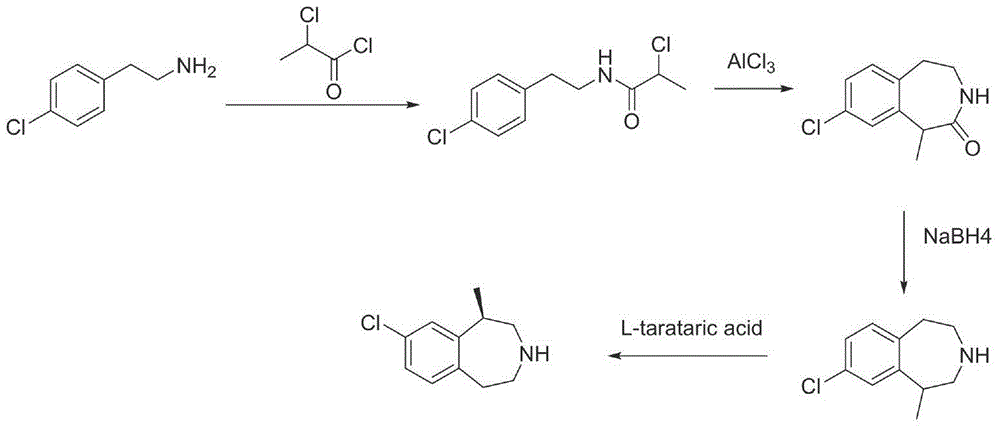
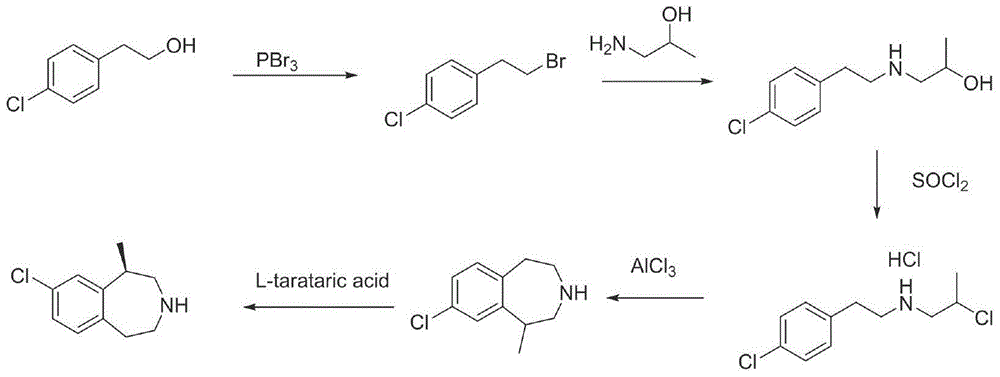
Preparation method of lorcaserin hydrochloride
A technology of lorcaserin hydrochloride and chlorophenylacetate, which is applied in the field of preparation of weight-loss drug lorcaserin hydrochloride, can solve the problems of long production cycle, harsh reaction requirements, high price, etc., and achieve reduction The production cycle, post-processing is simple, and the effect of low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] (1) Preparation of 2-(4-chlorophenyl)-N-(2-hydroxypropyl)acetamide formula (V)
[0041] Add 900ml of methanol and 82.6g (1.1mol) of isopropanolamine to a 3000ml three-necked flask, stir evenly, add 184.6g (1mol) of methyl p-chlorophenylacetate, heat to 50°C, keep warm for 6 hours, and recover the solvent under reduced pressure , add 5L of industrial water, crystallize at a temperature of 20-25°C, filter, and dry the filter cake to obtain 2-(4-chlorophenyl)-N-(2-hydroxypropyl)acetamide formula (V), white solid 223g , yield 97.9%.
[0042] (2) Preparation of 2-(4-chlorophenyl)-N-(2-chloropropyl)acetamide formula (IV)
[0043] Add 500ml of toluene and 114g (0.5mol) of 2-(4-chlorophenyl)-N-(2-hydroxypropyl)acetamide (V) into a 1000ml three-neck flask, stir to dissolve, and cool down to 0-5 in an ice-water bath. ℃, slowly add 90g (0.6mol) of thionyl chloride dropwise, after the dropwise addition, add 5ml of triethylamine, control the temperature at 10-20℃, react for 2-3 ho...
Embodiment 2
[0051] (1) Preparation of 2-(4-chlorophenyl)-N-(2-hydroxypropyl)acetamide formula (V)
[0052] Add 1000ml of ethanol and 82.6g (1.1mol) of isopropanolamine to a 3000ml three-neck flask, stir well, add 184.6g (1mol) of methyl p-chlorophenylacetate, heat to 70°C, keep warm for 4 hours, and recover the solvent under reduced pressure , add 5L of industrial water, crystallize at a temperature of 20-25°C, filter, and dry the filter cake to obtain 2-(4-chlorophenyl)-N-(2-hydroxypropyl)acetamide formula (V), white solid 224g , yield 98.3%.
[0053] (2) Preparation of 2-(4-chlorophenyl)-N-(2-chloropropyl)acetamide formula (IV)
[0054] Add 500ml of dichloromethane, 114g (0.5mol) of 2-(4-chlorophenyl)-N-(2-hydroxypropyl)acetamide (V) into a 1000ml three-necked flask, stir to dissolve, cool to 0 in an ice-water bath ~5°C, slowly add 52g (0.25mol) of phosphorus pentachloride, after the addition is complete, control the temperature at 30-40°C, react for 2-3 hours, recover the solvent, th...
Embodiment 3
[0062] (1) Preparation of 2-(4-chlorophenyl)-N-(2-hydroxypropyl)acetamide formula (V)
[0063] Add 1000ml of dioxane and 82.6g (1.1mol) of isopropanolamine to a 3000ml three-neck flask, stir evenly, add 184.6g (1mol) of methyl p-chlorophenylacetate, heat to 70°C, keep warm for 5 hours, reduce Recover the solvent under pressure, add 5L of industrial water, crystallize at a temperature of 20-25°C, filter, and dry the filter cake to obtain 2-(4-chlorophenyl)-N-(2-hydroxypropyl)acetamide formula (V), White solid 223.8g, yield 98.0%.
[0064] (2) Preparation of 2-(4-chlorophenyl)-N-(2-bromopropyl)acetamide formula (IV)
[0065] Add 500ml of dichloromethane, 114g (0.5mol) of 2-(4-chlorophenyl)-N-(2-hydroxypropyl)acetamide (V) into a 1000ml three-necked flask, stir to dissolve, cool to 0 in an ice-water bath ~5°C, slowly add 68g (0.25mol) of phosphorus tribromide, after the addition is complete, control the temperature at 30-40°C, react for 2-3 hours, recover the solvent, then add ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com