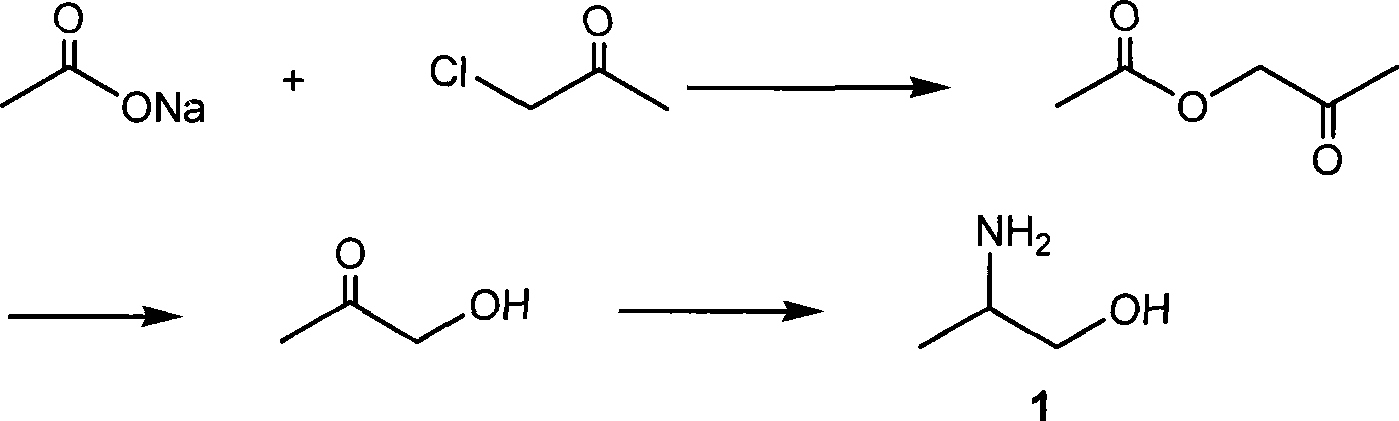
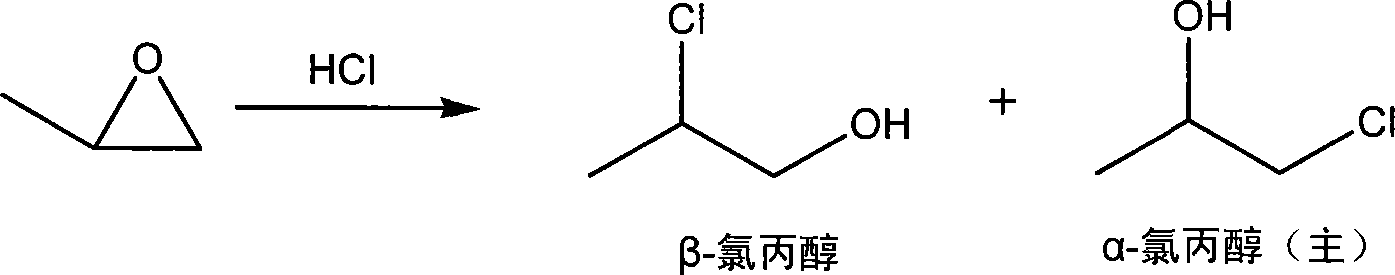
Method of synthesizing 2-aminopropanol
A technology of aminopropanol and synthesis method, which is applied in the preparation of aminohydroxyl compounds, chemical instruments and methods, and the preparation of organic compounds, etc., to achieve the effects of high atom economy, simple reaction, and cost reduction
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Embodiment 1: the preparation of β-chloropropanol
[0037] Add 370g of concentrated hydrochloric acid to 80g of water to form a hydrochloric acid aqueous solution with a mass concentration of about 30%, preheat to 50°C, start to add cold 200g of propylene oxide dropwise, drop directly to the liquid surface, and maintain the temperature at 55°C during the dropwise addition ~60°C, about 45 minutes to complete the dropwise addition, stir for 15 minutes, cool to room temperature, add solid anhydrous sodium carbonate to neutralize the reaction solution to pH = 7, leave to separate layers, the water layer is set aside, and the oil layer is dried with anhydrous sodium sulfate After filtering, the filtrate was rectified to collect 180.1 g of α-chloropropanol as a fraction at 127 to 130° C., and 122.0 g of β-chloropropanol as a fraction at 132 to 134° C.
[0038] Mix 85g of calcium hydroxide and 100g of water to form a lime milk suspension, stir and preheat to about 100°C, and a...
Embodiment 2
[0040] Embodiment 2: the preparation of 2-aminopropanol
[0041] Add 30g of β-chloropropanol and 0.5g of KI into the autoclave, replace the air in the autoclave with nitrogen for 3 times, then pass in about 100g of liquid ammonia to make the ammonia pressure in the autoclave 0.8MPa, gradually heat up with stirring, and keep the temperature at 130 ~140°C, stop the reaction after 20 hours, cool to room temperature, release ammonia gas, filter the reaction liquid to remove solids, and obtain 18.5 g of yellow liquid, namely 2-aminopropanol, with a yield of 77.7%.
Embodiment 3
[0042] Embodiment 3: the preparation of 2-aminopropanol
[0043] Add 94.5g of β-chloropropanol and 1g of KI into a high-pressure reaction kettle with a stirrer, replace the air in the kettle with nitrogen for 3 times, then pass in excess liquid ammonia, gradually heat up while stirring, and keep the temperature at 95°C for 30 hours. Stop the reaction, cool to room temperature, vent the ammonia gas, filter the reaction solution to remove solids, and obtain 58.2 g of light yellow liquid, namely 2-aminopropanol, with a yield of 77.6%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com