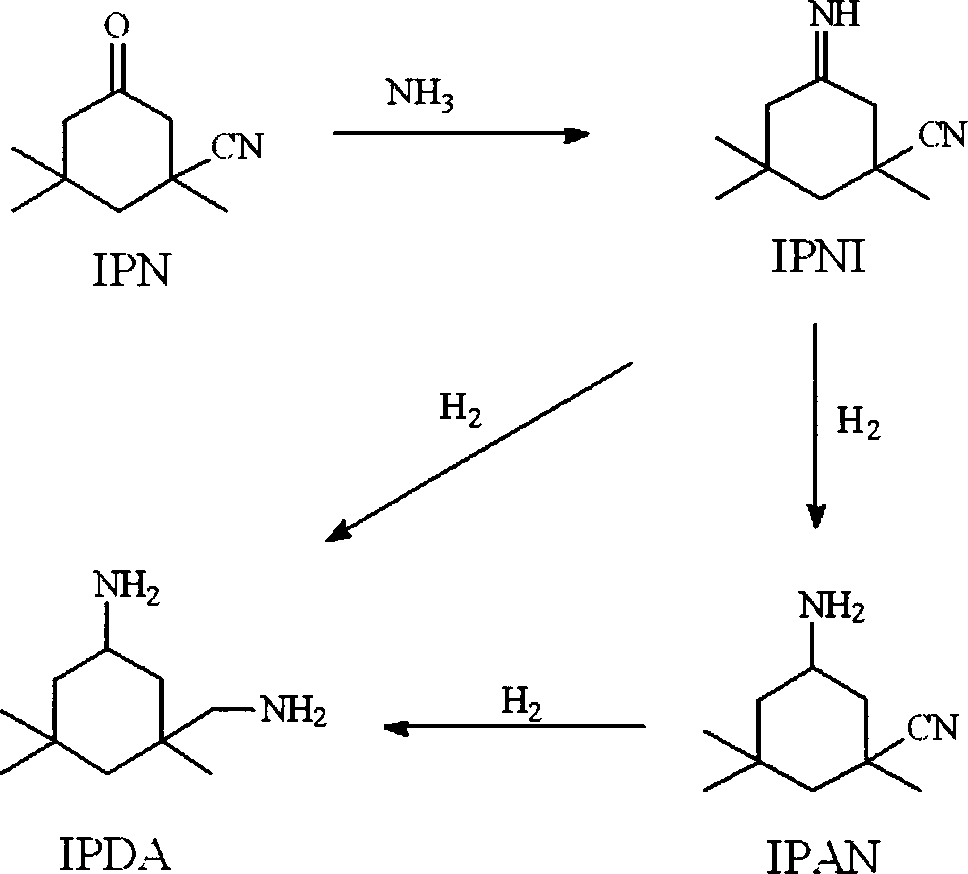
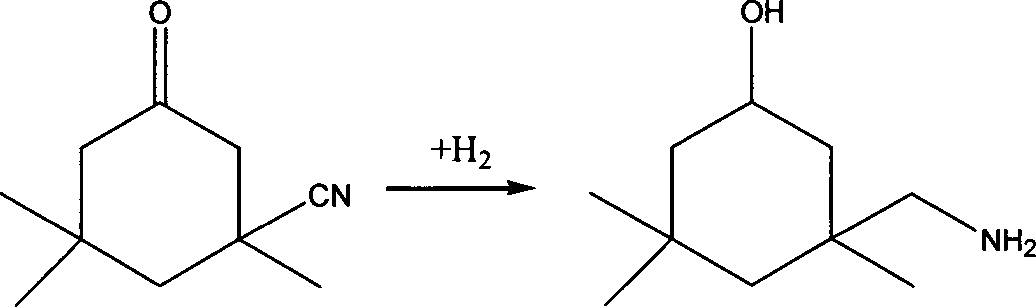Method for preparing 3-aminomethyl-3,5,5-trimethyl cyclohexylamine
A technology of trimethylcyclohexylamine and aminomethyl, which is applied in the field of preparation of aliphatic amines, can solve the problems of reduced production ratio, unstable cyano group, and intensified IPN decyanation reaction, so as to improve yield and operate simple effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
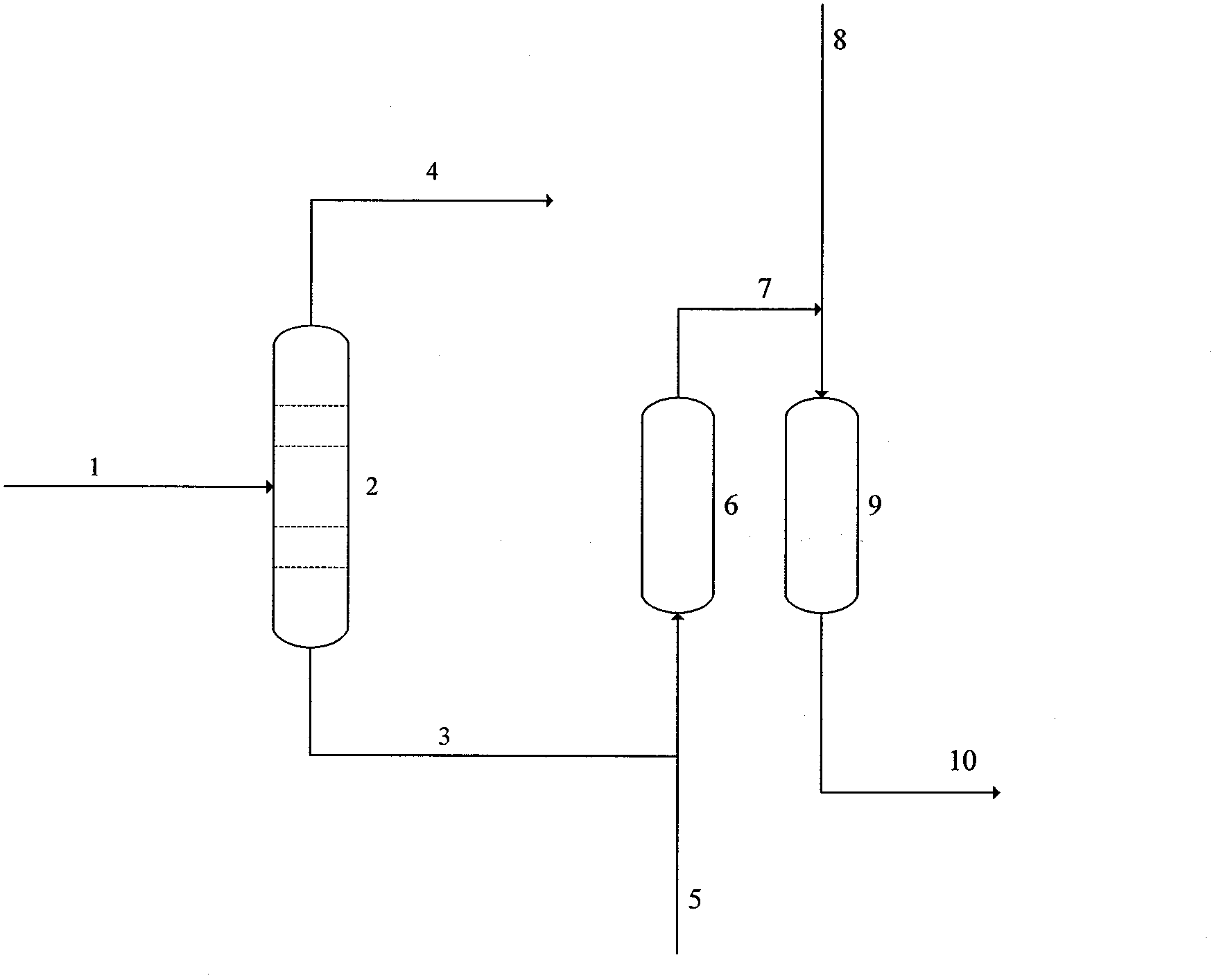
[0058] use as figure 1 The reaction setup shown was used for this example.
[0059] The reactive distillation tower (2) has an internal diameter of 40mm and a length of 1000mm, and is equipped with a 2mm-sized θ ring packing inside, and the feed inlet is located in the middle of the reactive distillation tower. The reactor (6) is 200mm in length and 25mm in inner diameter, and gamma-alumina pellets with a diameter of 1mm are housed in the reactor. The reactor (9) is 400 mm in length and 25 mm in inner diameter, and is equipped with a G62RS hydrogenation catalyst of 1 mm in diameter from Germany Southern Company.
[0060] In the first step, IPN enters the reactive distillation column (2) at 165g / h and IPDA at 510g / h from the middle of the reactive distillation column (2). The pressure of the reactive distillation column is controlled at 50Kpa by a vacuum pump, and the temperature of the bottom of the column is about 200°C , the top temperature is about 81°C.
[0061] In the ...
Embodiment 2-4
[0072] Carry out embodiment 2-4 according to the method identical with embodiment 1, just change the IPDA as primary amine in the first step into ethylenediamine, hexamethylenediamine and aniline respectively of the same molar amount.
[0073] The composition of the hydrogenation reaction product was also analyzed, and the results are shown in Table 2 below.
[0074] Table 2
[0075]
[0076] The main by-products IPAA and 3,3,5-trimethylcyclohexanol in the traditional method were not detected.
Embodiment 5-7
[0078] Carry out embodiment 5-7 according to the same method as embodiment 1, just change the ammonolysis catalyst in the second step into commercially available 1mm titanium dioxide pellets, silica pellets and ion exchange resins by gamma-alumina (Nankai University D72).
[0079] The composition of each product in the hydrogenation reaction product was also analyzed, and the results are shown in Table 3 below.
[0080] table 3
[0081]
[0082] The main by-products IPAA and 3,3,5-trimethylcyclohexanol in the traditional method were not detected.
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com