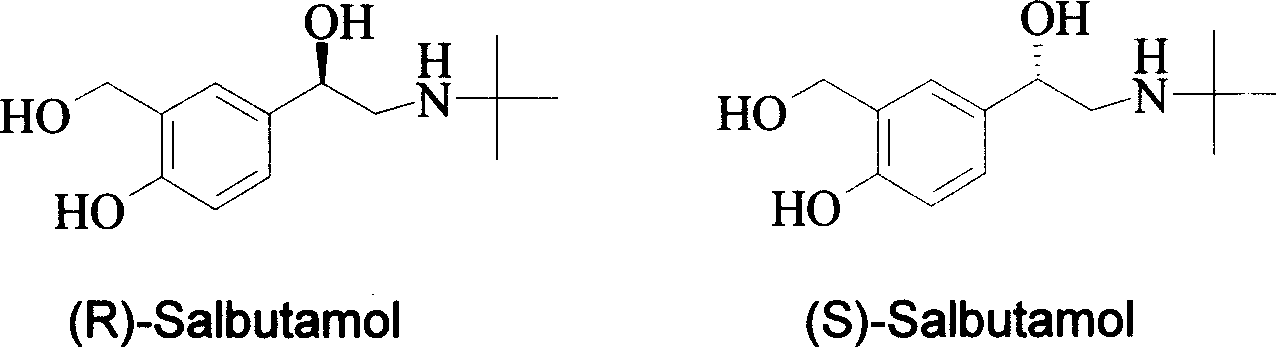Method for asymmetrical hydrogen transfer of alpha-imino keton for synthesizing chirality salbutamol
A technology for salbutamol and iminone, which is applied in the field of synthesis of chiral-beta amino alcohol compounds and achieves the effects of mild reaction conditions, simple processing and simple post-processing
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
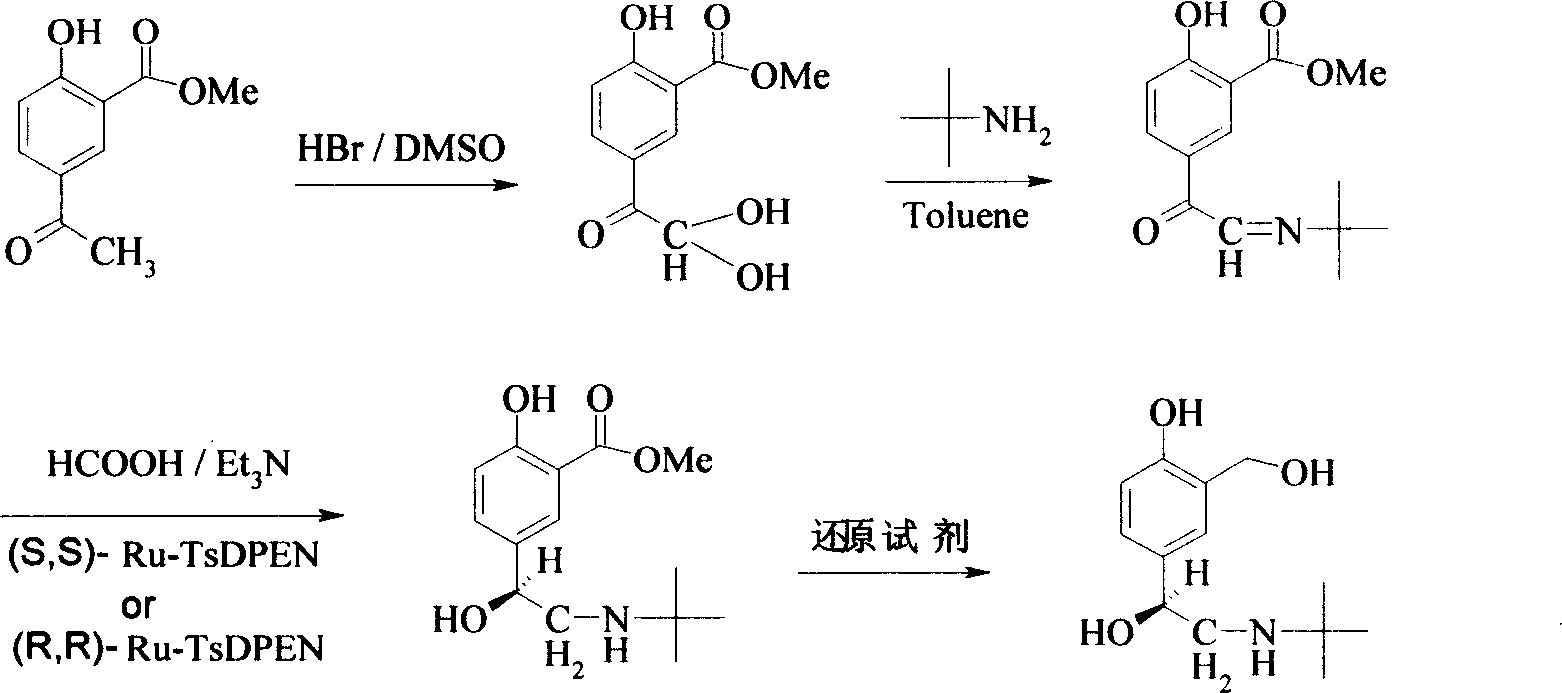
[0027] The first step is the preparation of methyl 5-(1,1-dihydroxy)-acetyl-2-hydroxybenzoate.
[0028] Add 75ml dimethyl sulfoxide (DMSO) in the 250ml three-necked bottle, 19.5g (0.1mol) methyl 5-acetyl salicylate, under room temperature, hydrobromic acid (HBr) 28ml (40%, 0.2mol, 2.0 eq) was added slowly, and the dropwise addition was completed in 40 minutes. The reaction solution was heated to 65° C., and reacted for about 20 hours. TLC detected that the raw material 5-acetyl salicylate methyl ester disappeared, and the reaction solution was slowly poured into 200 ml of ice water, and stirred for 30 Minutes, filtered, the solid was washed 3 times with 25ml of ice water, and vacuum-dried at room temperature to obtain 20.3g of the product 5-(1,1-dihydroxy)-acetyl-2-hydroxybenzoic acid methyl ester, with a yield of 90%. After further purification, it can be directly put into the next reaction.
[0029] The second step is the preparation of methyl 5-((1,1-dimethylethyl)imino)acet...
Embodiment 2
[0036] The first and second steps are the same as in Example 1.
[0037] The third step is the preparation of (S)-5-[2-[(1,1-dimethylethyl)amino]-1-hydroxyethyl]-2-hydroxybenzoic acid methyl ester.
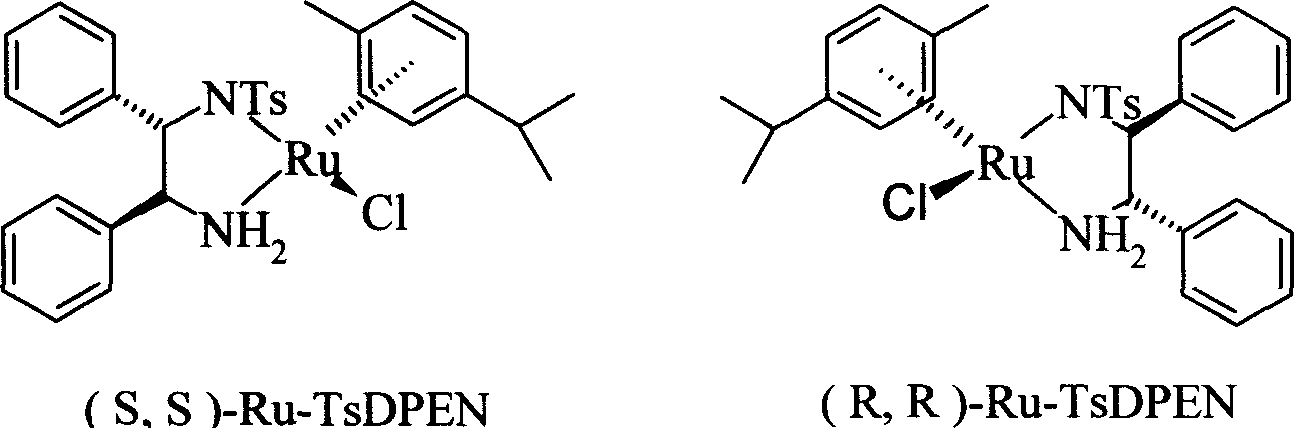
[0038] Under nitrogen protection, 0.26g (1mmol) of the compound obtained in the second step, (R, R)-Ru-TsDPEN6.4mg (0.01mmol), Et 3 Dissolve N 0.69ml (5.0mmol) in 2.0ml DMF, add HCOOH 0.07ml (2.0mmol) slowly, stir at room temperature for 24 hours, pour the reaction solution into 5.0ml water, extract twice with 10ml ethyl acetate, anhydrous sodium sulfate Drying, solvent evaporation under reduced pressure, and column chromatography to obtain the product (S)-5-[2-[(1,1-dimethylethyl)amino]-1-hydroxyethyl]-2-hydroxybenzoic acid Methyl ester 0.13g, 96%ee, yield 48%.
[0039] The fourth step (S)-Salbutamol is: the preparation of (S)-2-(nitrogen-tert-butylamino)-1-(4-hydroxyl-3-hydroxymethylphenyl)ethanol
[0040] Under nitrogen protection condition, the product (S)-5-[2-[(1,1-dimeth...
Embodiment 3
[0042] The first, second and third steps are the same as in Example 1.
[0043] The fourth step is the preparation of (R)-Salbutamol: (R)-2-(nitrogen-tert-butylamino)-1-(4-hydroxyl-3-hydroxymethylphenyl)ethanol.
[0044] Under nitrogen protection condition, take by weighing the product 0.27g (1mmol) that the 3rd step makes, NaBH 4 95mg (2.5mmol), suspended in 10ml of anhydrous tetrahydrofuran (THF), slowly dropwise added 0.4ml of BF3.Et2O solution (content 47%, d, 1.13, 1.5mmol) at room temperature, and heated the reaction solution to 55°C after the dropwise addition React for 3 hours, drop to room temperature, drop methanol 4ml, then heat the reaction solution to 55°C for 1.5 hours, cool to room temperature, add N,N,N,N-tetramethylethylenediamine (TMEDA) 0.23ml ( 1.5mmol), stirred at room temperature for 6 hours and then filtered, the solvent was evaporated under reduced pressure, and the product (R)-Salbutamol was obtained by column chromatography was 0.18g, 98% ee, and th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com