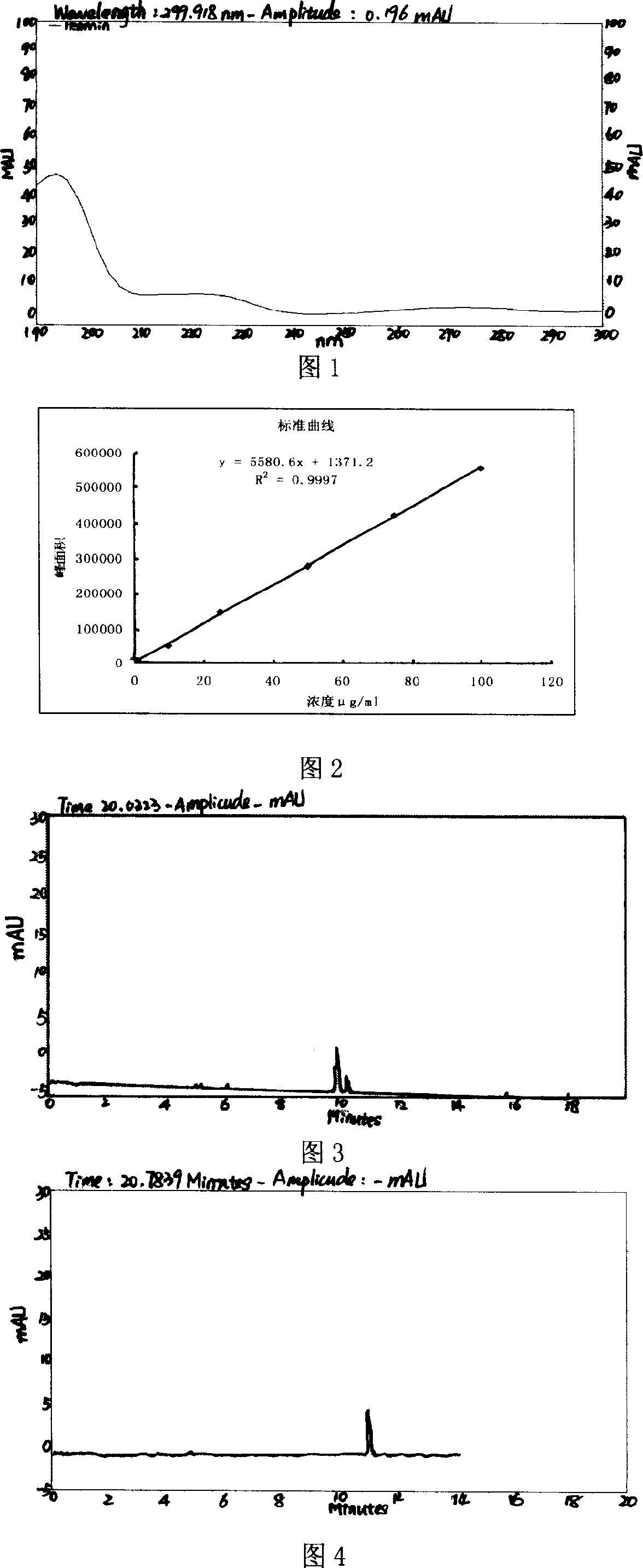
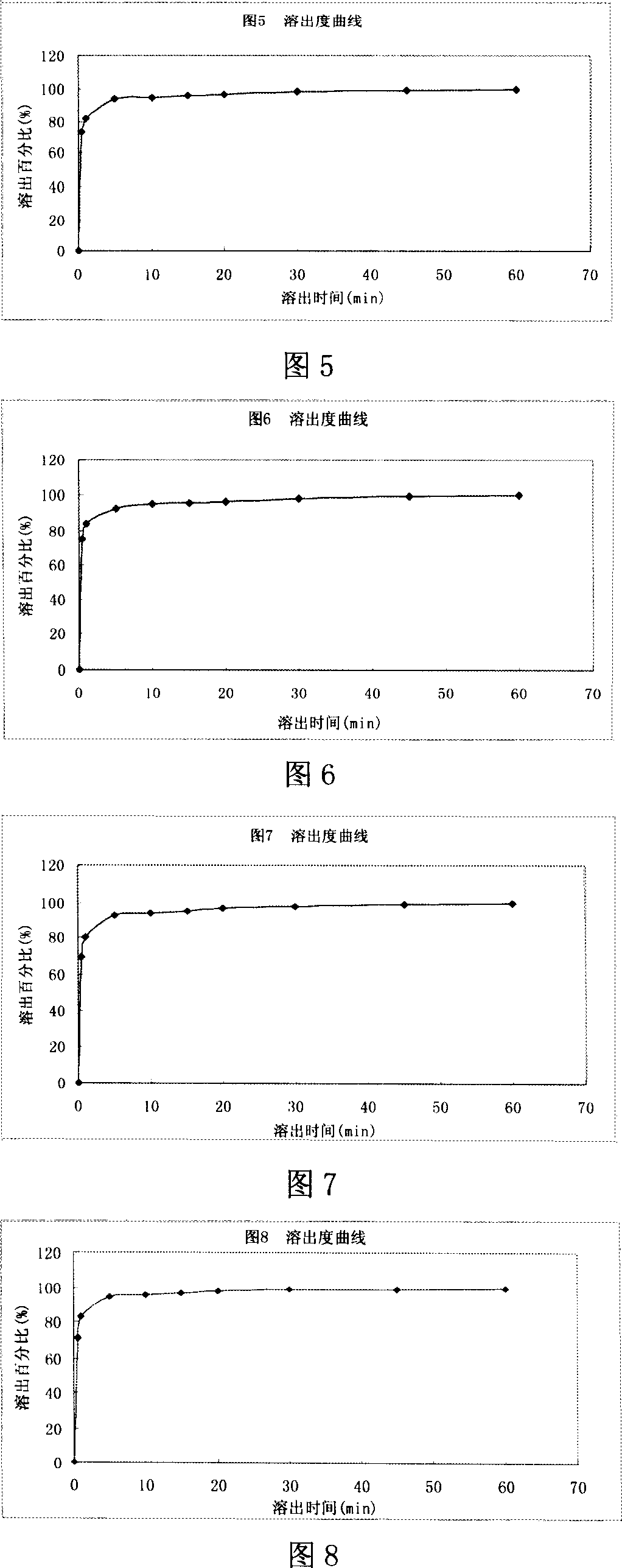
Levalbuterol hydrochloride orally disintegrating tablet and preparation method thereof
A technology of levalbuterol hydrochloride and oral fast disintegrating tablets, which is applied in the directions of pharmaceutical formulations, medical preparations with inactive ingredients, and medical preparations containing active ingredients, etc. Optically active drug failure and other problems, to achieve the effect of low cost, simple process, good disintegration performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0069] The prescription of levosalbutamol hydrochloride orally disintegrating tablets in this example is: 1.26 parts of levosalbutamol hydrochloride, 2.52 parts of β-cyclodextrin, 28.51 parts of microcrystalline cellulose, 14.25 parts of low-substituted hydroxypropyl cellulose, cross-linked polyethylene 14.25 parts of pyrrolidone, 28.51 parts of mannitol, 5.35 parts of citric acid, and 5.35 parts of aspartame.
[0070] The preparation method of levosalbutamol hydrochloride oral fast disintegrating tablet is:
[0071] Weigh levosalbutamol hydrochloride, β-cyclodextrin, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, cross-linked polyvinylpyrrolidone, mannitol, citric acid, and aspartame according to the amount, and pass through an 80-mesh sieve , mix and grind levosalbutamol hydrochloride and β-cyclodextrin fully for 20 minutes, mix levosalbutamol hydrochloride β-cyclodextrin inclusion complex with disintegrating agent, filler and flavoring agent evenly acc...
Embodiment 2
[0095] The prescription of levosalbutamol hydrochloride orally disintegrating tablets in this example is: 2.10 parts of levosalbutamol hydrochloride, 6.30 parts of β-cyclodextrin, 37.44 parts of microcrystalline cellulose, 6.18 parts of low-substituted hydroxypropyl cellulose, cross-linked polyethylene 14.54 parts of pyrrolidone, 21.8 parts of lactose, 5.82 parts of citric acid, 5.82 parts of aspartame.
[0096] The preparation method of levosalbutamol hydrochloride oral fast disintegrating tablet is:
[0097] Levosalbutamol hydrochloride, β-cyclodextrin, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, cross-linked polyvinylpyrrolidone, lactose, citric acid, and aspartame were weighed according to the amount, passed through a 80-mesh sieve, Mix levosalbutamol hydrochloride and β-cyclodextrin and grind them thoroughly for 30 minutes, mix levosalbutamol hydrochloride β-cyclodextrin inclusion complex with disintegrant, filler and flavoring agent uniformly acc...
Embodiment 3
[0118] The prescription of levosalbutamol hydrochloride orally disintegrating tablets in this example is: 0.79 parts of levosalbutamol hydrochloride, 3.15 parts of β-cyclodextrin, 35.32 parts of microcrystalline cellulose, 7.06 parts of low-substituted hydroxypropyl cellulose, cross-linked polyethylene 14.13 parts of pyrrolidone, 9.42 parts of mannitol, 18.83 parts of lactose, 5.65 parts of citric acid, and 5.65 parts of aspartame.
[0119] The preparation method of levosalbutamol hydrochloride oral fast disintegrating tablet is:
[0120] Weigh levosalbutamol hydrochloride, β-cyclodextrin, microcrystalline cellulose, low-substituted hydroxypropyl cellulose, cross-linked polyvinylpyrrolidone, mannitol, lactose, citric acid, and aspartame according to the amount, and pass 80 Mesh sieve, mix and grind levosalbutamol hydrochloride and β-cyclodextrin fully for 30 minutes, mix levosalbutamol hydrochloride β-cyclodextrin inclusion complex with disintegrating agent, filler and flavori...
PUM
| Property | Measurement | Unit |
|---|---|---|
| hardness | aaaaa | aaaaa |
| hardness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com