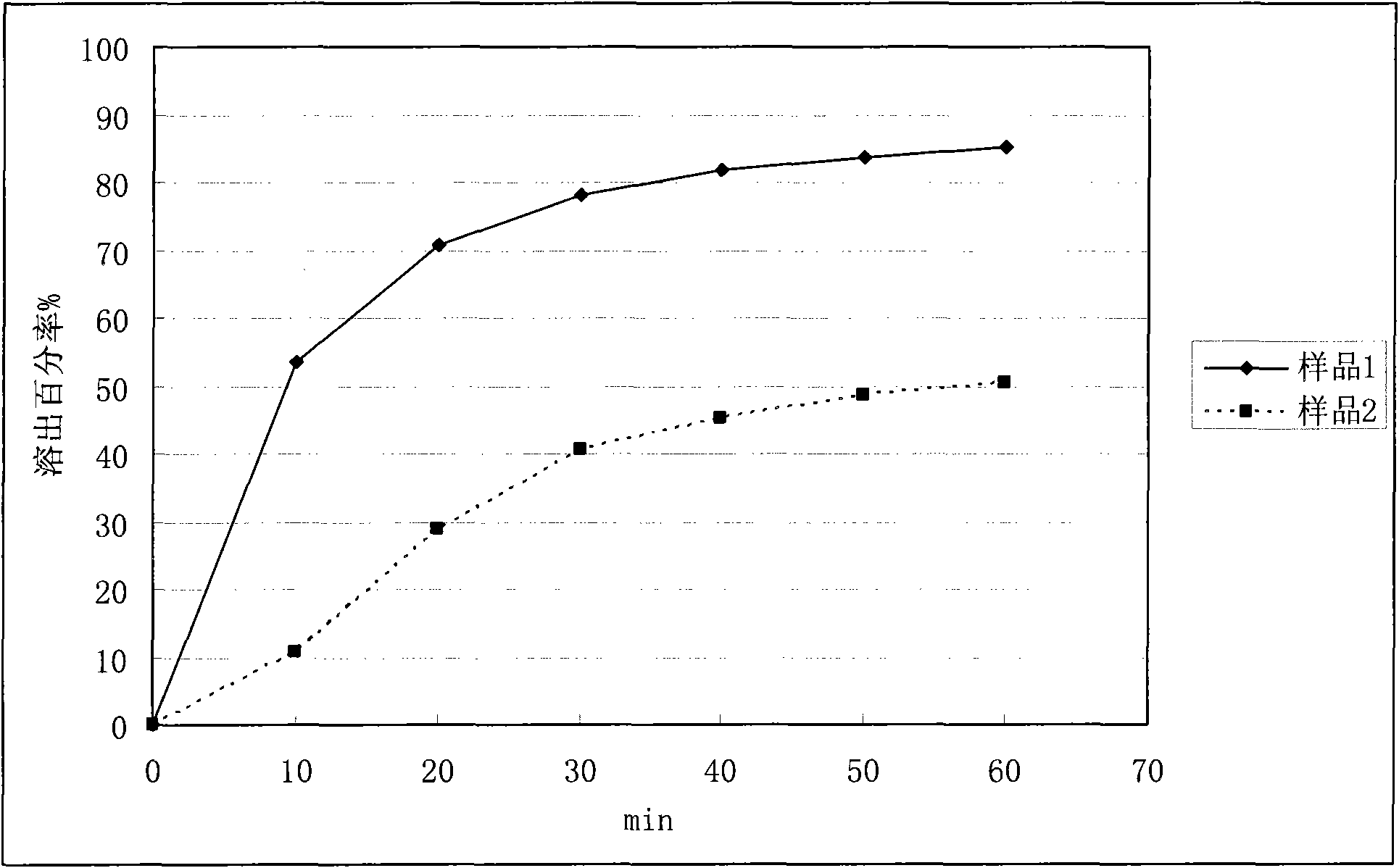
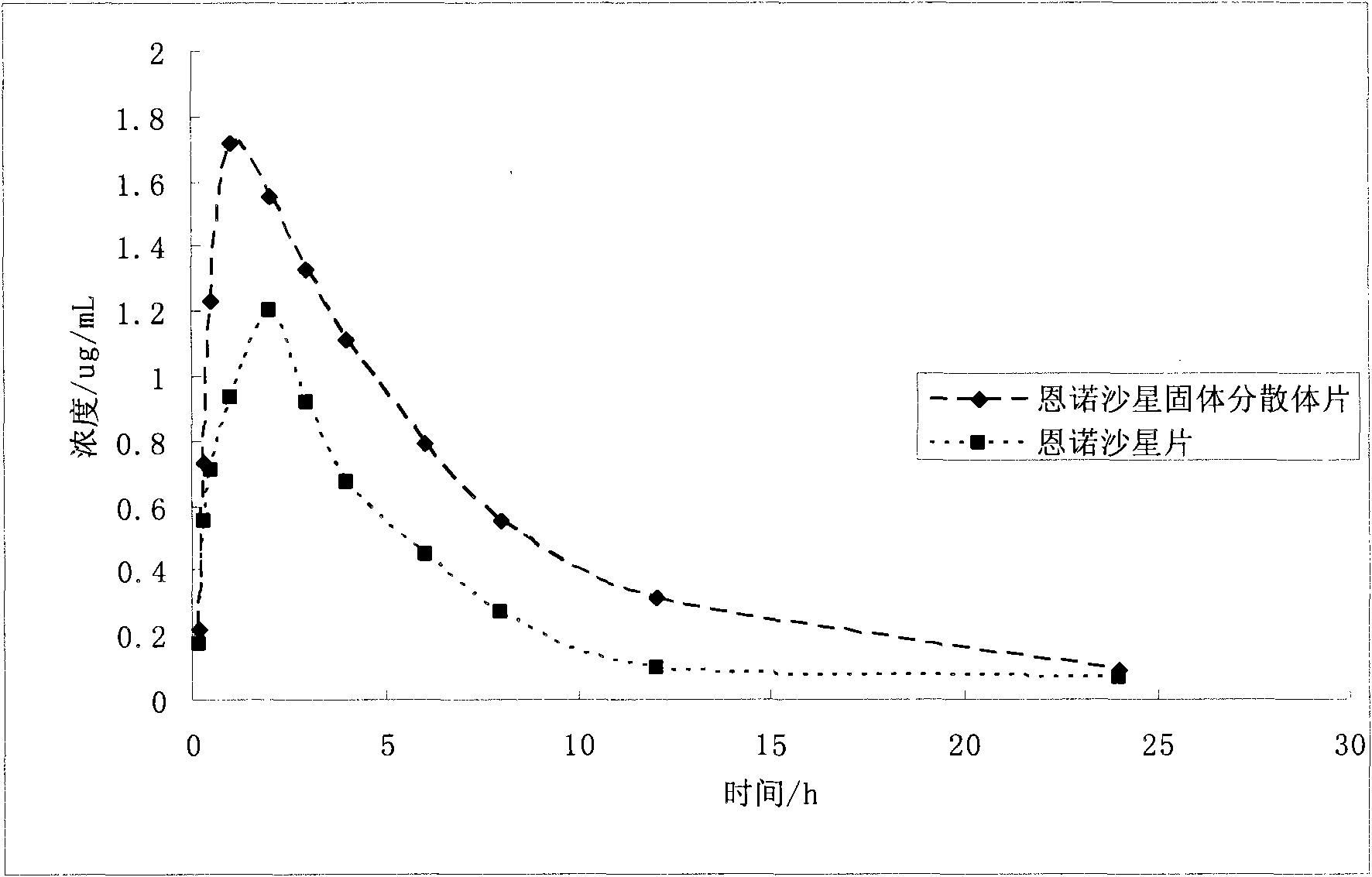
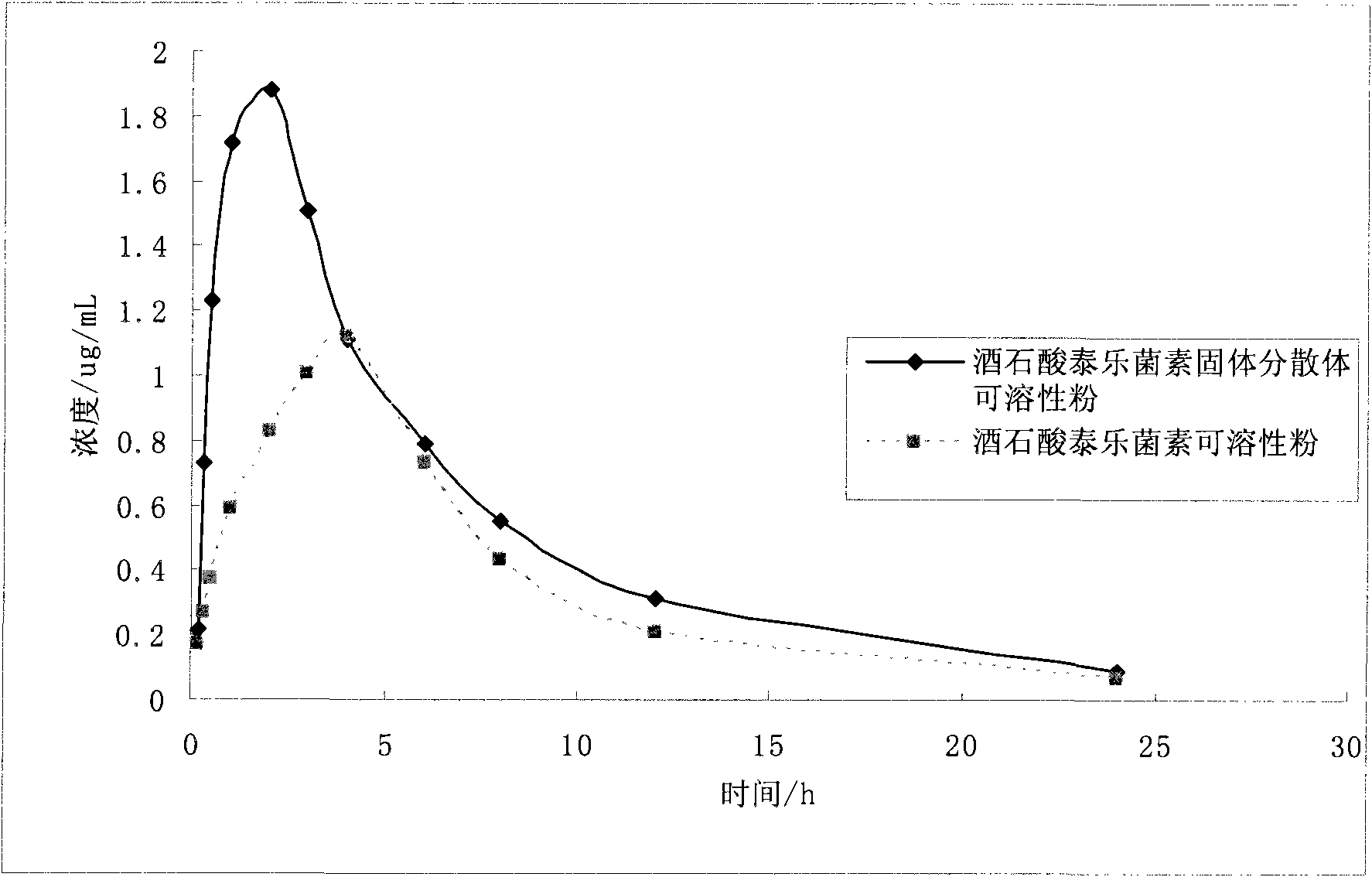
Application of solid dispersion to preparation of veterinary drugs
A solid dispersion and veterinary drug technology, applied in solid dispersion and its preparation, the application field of solid dispersion in veterinary drug preparation, can solve the problems of low drug bioavailability, low dissolution rate, breeding loss, etc., and achieve improved stability Sexuality and bioavailability, good dissolution rate, and the effect of improving the initial dissolution rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] The preparation method of the capsules of the present invention is as follows: adding pharmaceutical auxiliary materials to the solid dispersion, mixing them, processing them into powders or granules, and packing them into hard gelatin capsule shells;
[0037] The solid dispersion of the present invention can also be prepared as a premix capsule, which is prepared by adding pharmaceutical adjuvants and corncob powder, bean cake powder, soybean meal powder, bran, rice bran or corn flour to the solid dispersion of the present invention ;
[0038] In the examples of the present invention, the preparation method of preparing the solid dispersion as an injection is given, adding pharmaceutical excipients to the solid dispersion, and processing it into an injection.
[0039] The pharmaceutical adjuvant of the oral dosage form in the embodiment of the present invention is the adjuvant commonly used in this field such as sucrose, dextrin, starch, the pharmaceutical adjuvant of ...
Embodiment 1
[0042] Embodiment 1 Preparation and properties of enrofloxacin solid dispersion and preparation thereof
[0043] 1. Enrofloxacin solid dispersion and its preparation
[0044] The enrofloxacin solid dispersion contains 20% enrofloxacin and 80% PVPk15.
[0045] Enrofloxacin is soluble in organic solvents, so the solvent-spray drying method was chosen:
[0046] Dissolve 20g of enrofloxacin and 80g of PVPk15 in 1000ml of methanol-chloroform (volume ratio 1:5) mixed solution, mix well, spray dry the solution, and place it in a vacuum oven at 40°C-60°C for 2-3 hours , Wipe off a small amount of residual solvent, that is, get enrofloxacin solid dispersion.
[0047] 2. Dissolution test of enrofloxacin solid dispersion
[0048] 1) Determination of Enrofloxacin Content
[0049] Chromatographic conditions: Octadecylsilane bonded silica gel is used as filler; column temperature is 40°C; detection wavelength is 278nm; theoretical plate number should not be less than 2000 based on enrof...
Embodiment 2
[0089] Example 2 Preparation and properties of tylosin tartrate solid dispersion and medicine thereof
[0090] 1.32.5% W / W tylosin tartrate solid dispersion and preparation method thereof
[0091] The 32.5% tylosin tartrate solid dispersion contains 32.5% tylosin tartrate and 67.5% PEG4000.
[0092] The preparation of 32.5% tylosin tartrate solid dispersion, because tylosin tartrate is insoluble in organic solvents, can choose the grinding method or melting method, this embodiment chooses to use the melting method to prepare, the method is as follows:
[0093] Take 67.5g of PEG4000 and heat it to melt, then add 32.5g of the active ingredient tylosin tartrate, stir while adding, until the tylosin tartrate is completely dissolved, immediately pour it into a low-temperature container at -30°C ± 5°C to cool, and keep it in the freezer After 60 minutes, take it out, transfer it to a desiccator for drying, crush it and pass it through an 80-120 mesh sieve to obtain a solid dispersi...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com