Cell Population Having Immunoregulatory Activity, Method for Isolation and Uses
a cell population and immunoregulatory technology, applied in the field of cell population having immunoregulatory activity, method for isolation and use, can solve the problems of affecting the survival of patients, causing death, and causing harm or deadly immunological responses, so as to prevent, treat or improve the
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
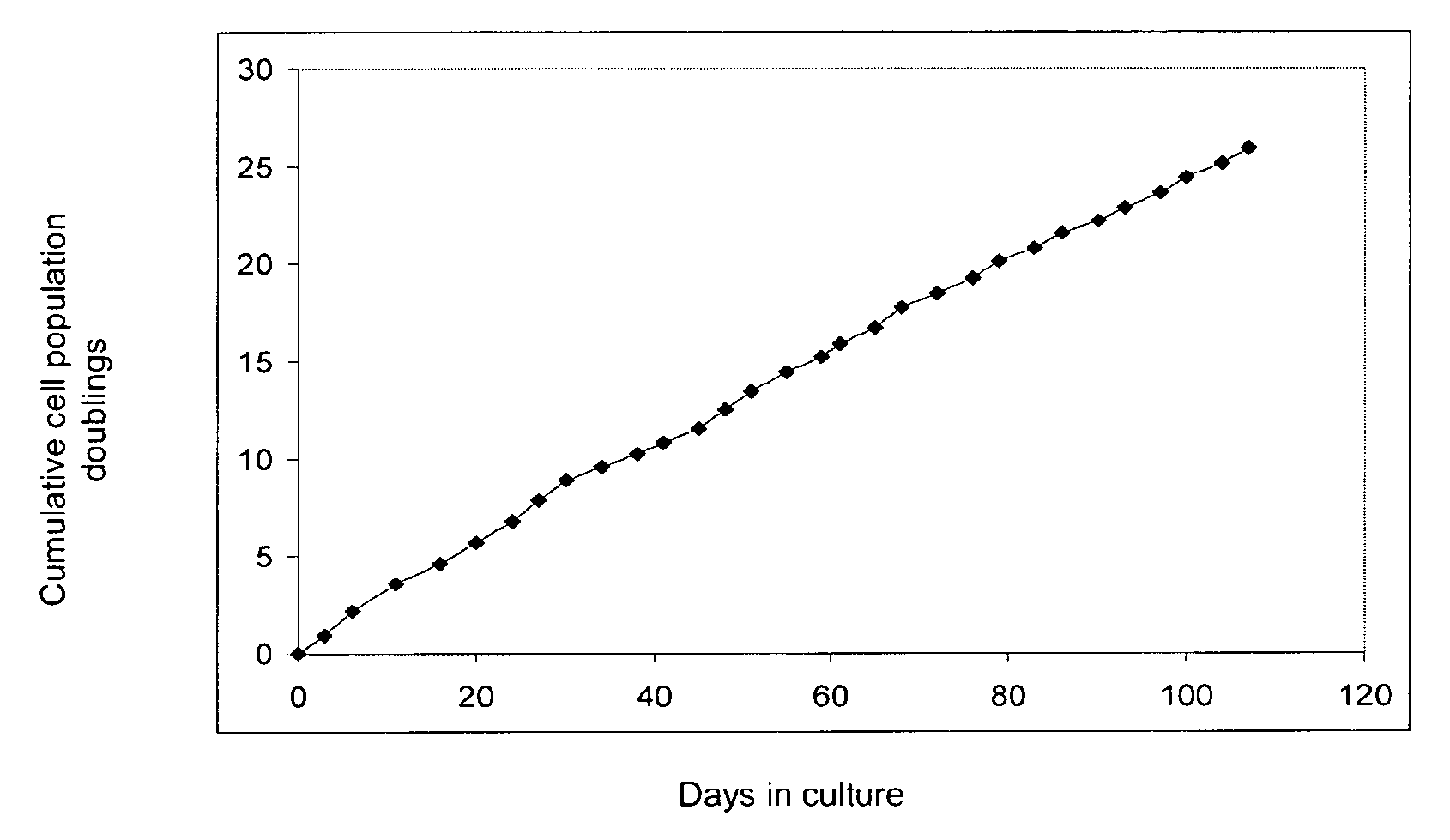
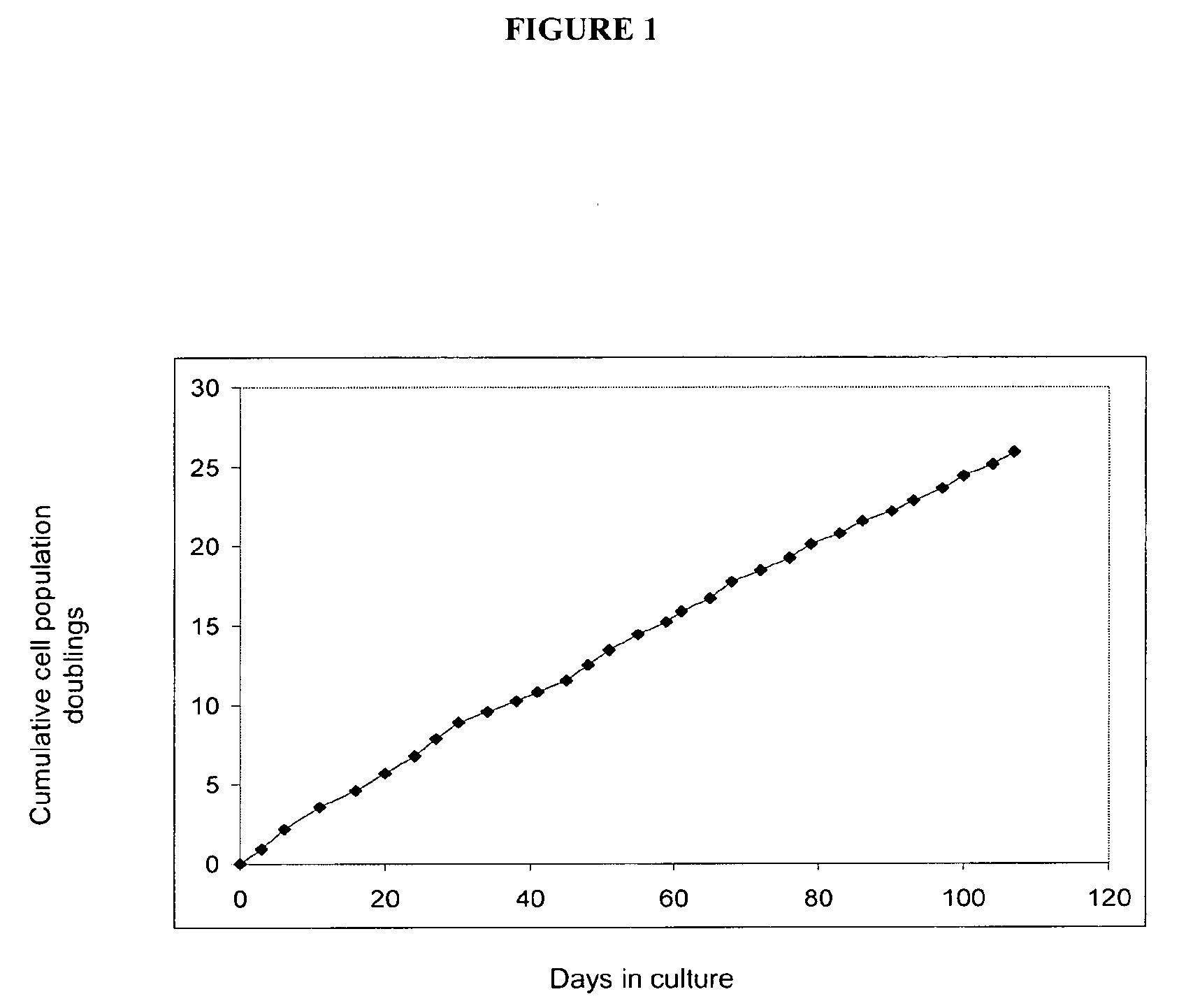
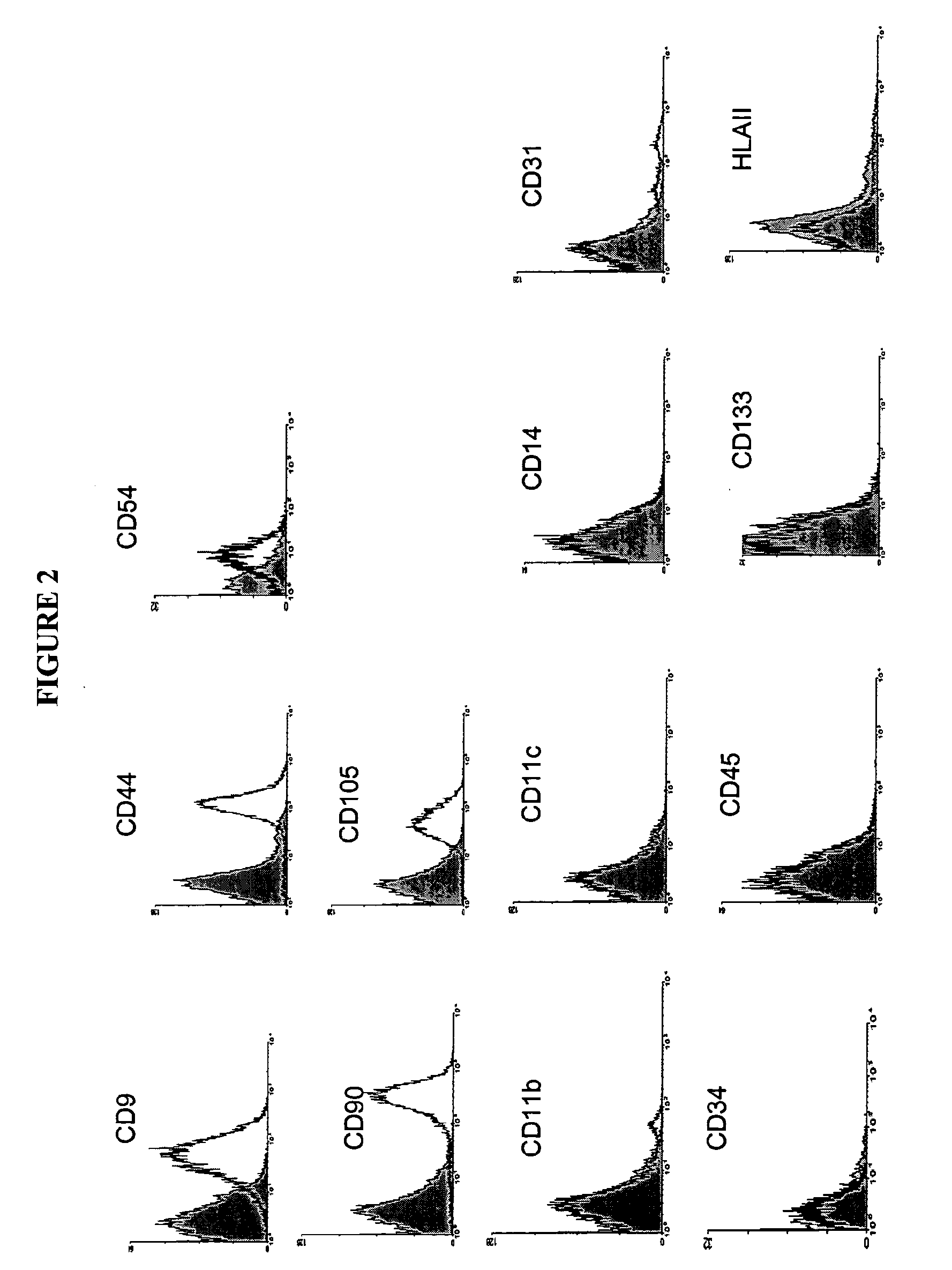
Image
Examples
example 1
Isolation and Expansion of Cells of the Invention
I. Material and Methods
[0187]Isolation of Cells of the Invention from Adipose Tissue
[0188]Human adipose tissue was obtained by liposuction, under local anaesthesia and general sedation. A hollow blunt-tipped cannula was introduced into the subcutaneous space through a small incision (less than 0.5 cm in diameter). With gentle suction, the cannula was moved through the adipose tissue abdominal-wall compartment for mechanical disruption of the fatty tissue. A saline solution and the vasoconstrictor epinephrine were injected into the adipose tissue compartment to minimize blood loss. In this way, 80 to 100 ml of raw lipoaspirate were obtained from each patient to be treated.
[0189]The raw lipoaspirate was washed extensively with sterile phosphate-buffered saline (PBS; Gibco BRL, Paisley, Scotland, UK) to remove blood cells, saline and local anaesthetic. The extracellular matrix was digested with a solution of type II collagenase (0.075%; ...
example 2
Induction of Indolamine 2,3-dioxygenase (IDO) by interferon-gamma (IFN-γ)
I. Material and Methods
[0212]The cells of the invention isolated from human adipose tissue (Example 1), were seeded onto tissue culture plates at a density of 10,000 cells / cm2, and incubated for 48 hours in the conditions previously described for cell expansion. Then, different pro-inflammatory stimuli were added to the culture medium, including:[0213]Interleukin-1 (IL-1): 3 ng / ml[0214]Interferon-gamma (IFN-γ): 3 ng / ml[0215]Tumor necrosis factor-alpha (TNF-α): 5 ng / ml[0216]Lipopolysaccharide (LPS): 100 ng / ml
[0217]The cells were incubated in the presence of the corresponding stimulus for periods ranging form 30 minutes to 48 hours, and then they were collected by trypsin digestion, and lysed in RIPA buffer (50 mM Tris-HCl pH 7.4, 150 mM NaCl, 1 mM PMSF (phenyl-methylsulphonylfluoride), 1 mM EDTA (ethylenediaminetetraacetic acid), 5 μg / ml Aprotinin, 5 μg / ml Leupeptin, 1% Triton x-100, 1% Sodium deoxycholate, 0.1%...
example 3
Tumorigenic Behaviour
I. Material and Methods
[0220]This experiment was performed with cells of the invention isolated from human adipose tissue as described in Example 1. The cell samples were cultivated for 2-7 weeks prior to the subcutaneous implantation in immunodeficient mice (5×106 cells / mouse). The mice were nu / nu strain obtained from Charles River Laboratories. Mice lacked thymus and were T-cell deficient. The implanted mice were followed-up for 4 months prior to sacrifice and pathological study.
[0221]Pathological study: A necropsy was performed on all animals. The animals were examined for gross abnormalities in the brain, lungs, heart, liver, kidneys, spleen, abdominal lymph nodes and injection site. Tissues were collected for a histological examination (parafin section and hematoxilin-eosin (H&E) staining), including injection site, lungs and lymph nodes.
[0222]The teratome cellular line (N-TERA) was used as a positive control, which was implanted under identical conditions....
PUM
| Property | Measurement | Unit |
|---|---|---|
| Cell angle | aaaaa | aaaaa |
| Antigenicity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com