Treatment of hepatitis c in the asian population with interferon-beta
A technology of interferon and population, applied in the field of viral diseases, can solve problems such as poor clinical efficacy
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0127] Example 1: Interferon-beta-1a (Liby ) early viral response to treatment: 12-week results of a multicenter, randomized, placebo-controlled trial
[0128] Purpose
[0129] The primary purpose of this study was to evaluate and compare the effect of IFN-β-1a versus placebo on sustained viral response (SVR) in Asian patients with chronic hepatitis C infection not treated with IFN-α.
[0130] The second main objective of this study was to evaluate and compare the effect of interferon-β-1a and interferon-α-1a / ribavirin combination on SVR.
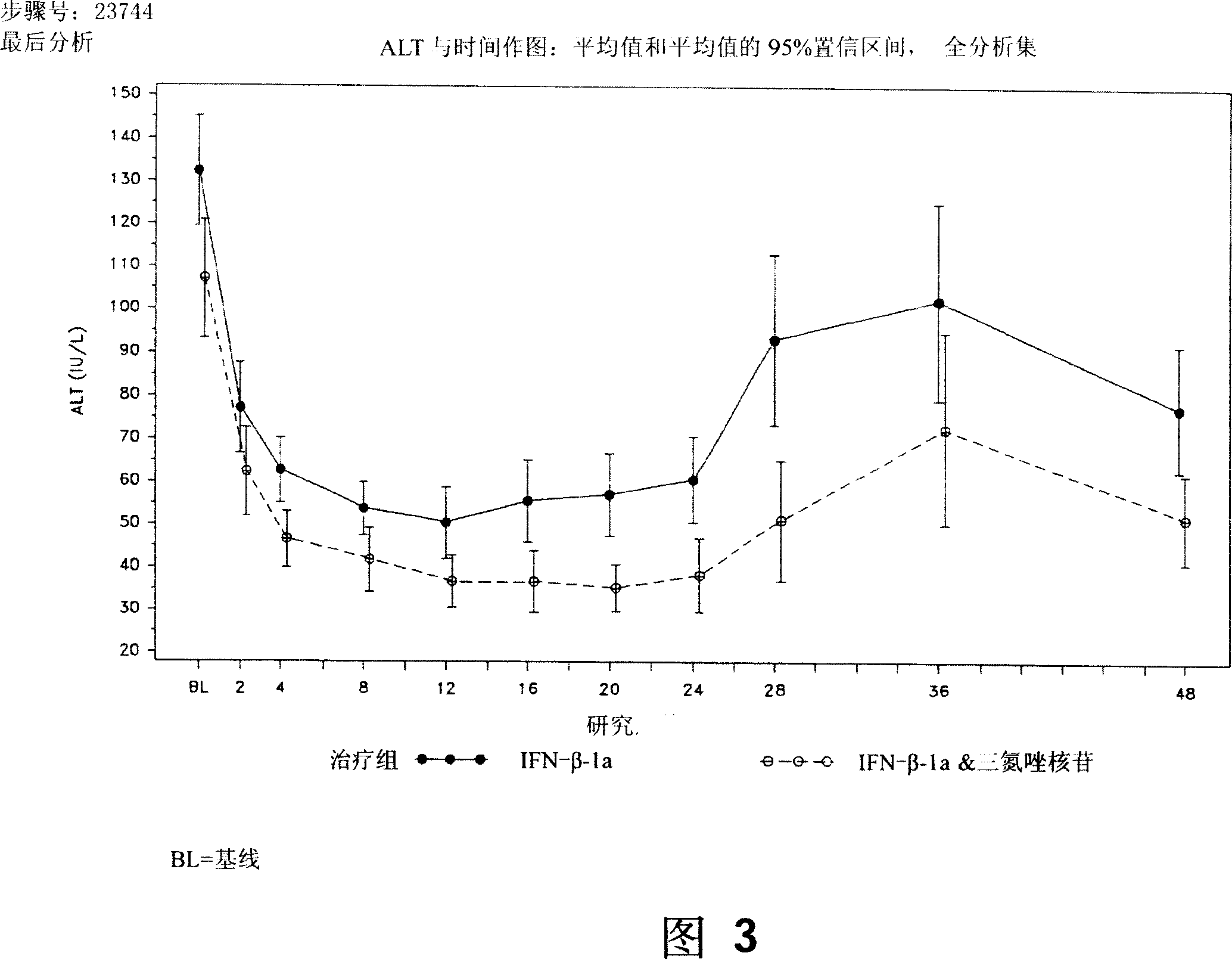
[0131]Another objective of this study was to evaluate and compare the effects of interferon-beta-1a on therapeutic viral load and clearance, alanine aminotransferase (ALT) levels, liver histology, and treatment safety and tolerability, including effects on hematological and biochemical parameters and development of neutralizing antibodies.
[0132] The present example presents the results of an analysis of the first 12 weeks of treatme...
Embodiment 2
[0187] Example 2 Sustained Viral Response to Interferon-beta-1a (Liby(R)) Treatment in Asian Hepatitis C Patients: 24 and 48 Week Results of a Multicenter, Randomized, and Placebo-Controlled Trial
[0188] Study Design and Materials and Methods:
[0189] The research design and materials and methods used are as in Example 1.
[0190] result:
[0191] Patient demographics and baseline disease characteristics are as in Example 1.
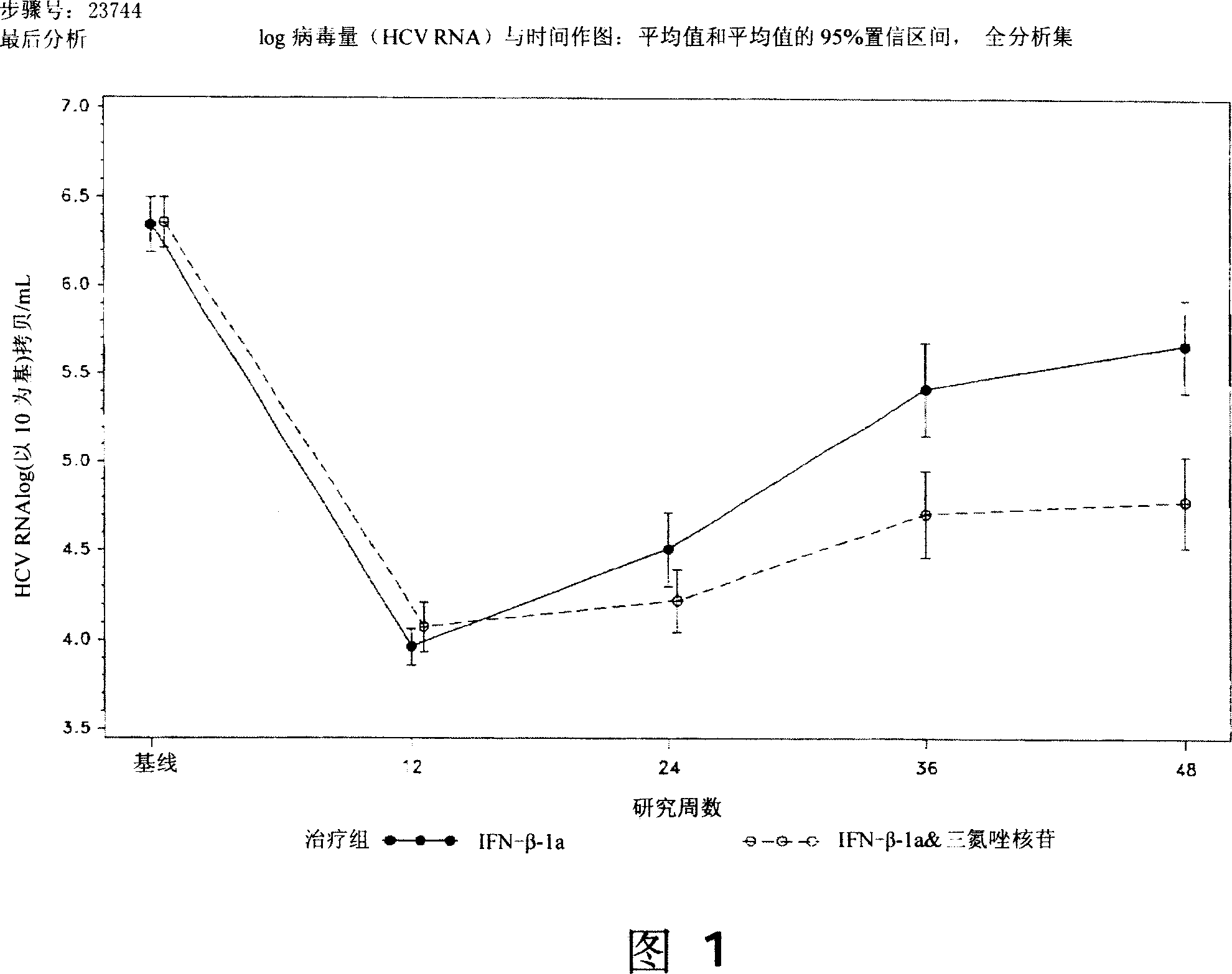
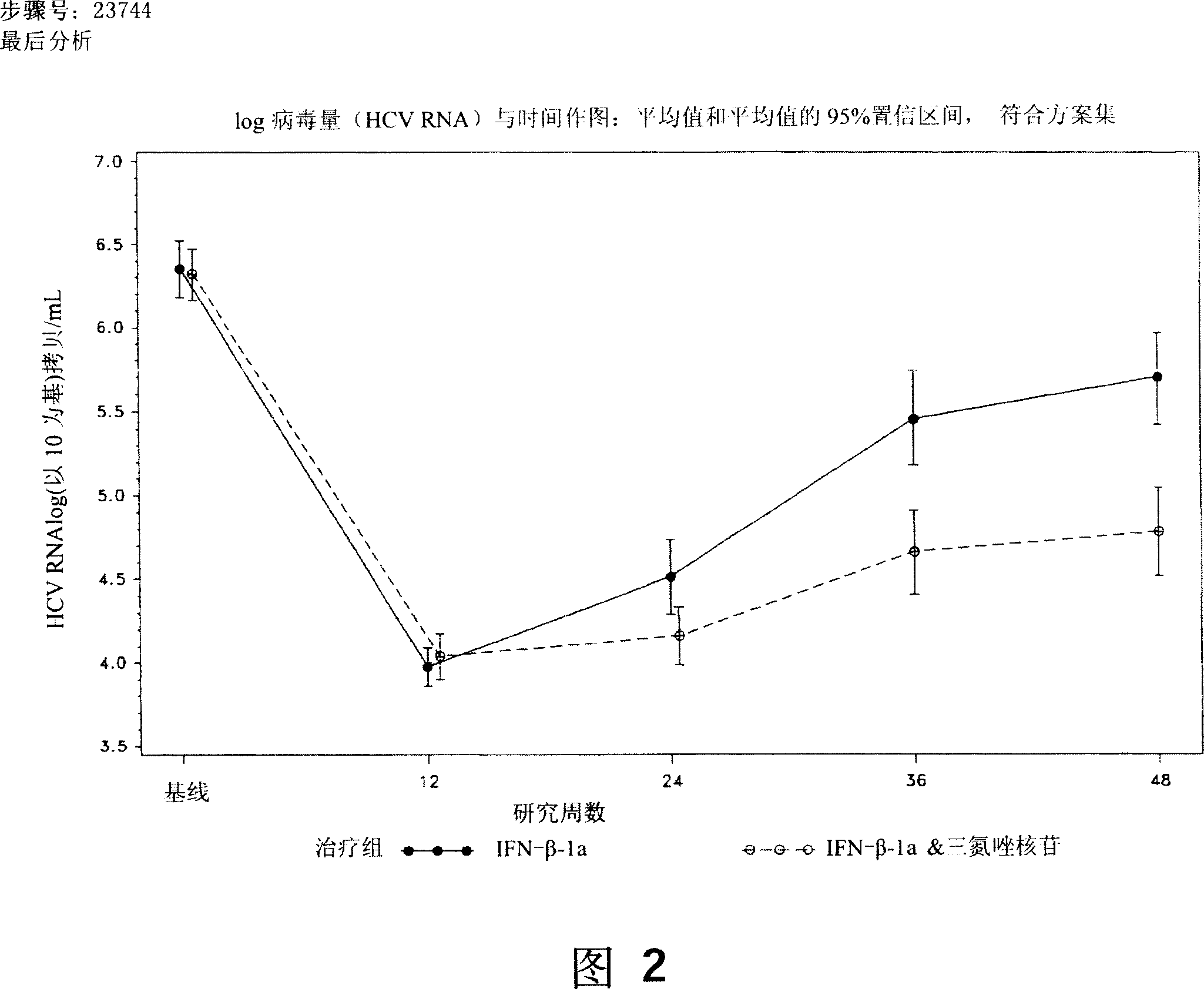
[0192] 128 subjects received interferon-beta-1a, 15 (11.7%) subjects withdrew before 48 weeks. All study subjects who received placebo during the double-blind period completed the first 12 weeks of the study. Two power analysis sets are analyzed: the full analysis set and the per-protocol set. The full analysis set was defined as all study subjects randomized and treated (ie, receiving at least one study drug) with at least one efficacy or safety value for a treatment-based measure. Subjects with no viral load (HCV RNA) at week 12 were also inclu...
Embodiment 3
[0212] Example 3 Combination therapy of interferon-beta-1a (Ribi(R)) and ribavirin in Asian patients with hepatitis C: 24-week and 48-week results of a multicentre, randomized and placebo-controlled trial
[0213] The final experimental data analysis has been carried out, and the results of the IFN-β and ribavirin combination treatment group in the draft statistical report are shown below.
[0214] Table 9 Week 48 SVR Summary, Full Analysis Set
[0215] IFN-β-1a
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com