Freeze-dried voriconazole micelle preparation and preparation method thereof
A technology of voriconazole and freeze-dried gel, which is applied in the field of voriconazole freeze-dried micellar preparations and its preparation, can solve the problems of no clinical application value, large freeze-dried volume, long production cycle, etc., and achieve avoidance of toxic effects and biocompatibility Good performance and fast reconstitution
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
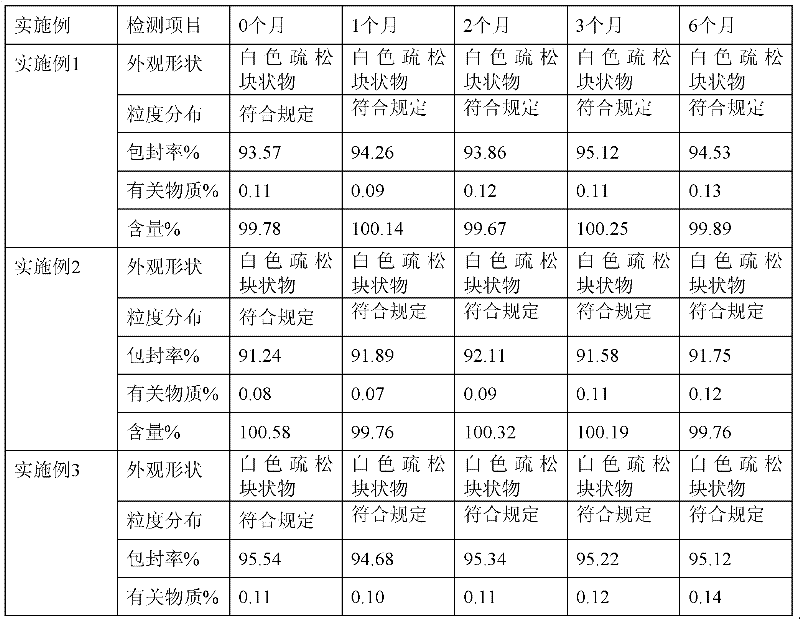
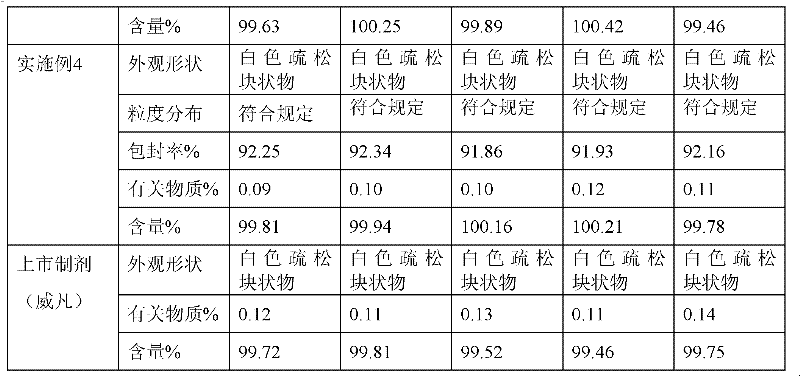
Embodiment 1
[0034] The preparation of embodiment 1 voriconazole lyophilized micellar preparation
[0035] Weigh voriconazole 200mg, sodium glycocholate 300mg, sodium taurodeoxycholate 150mg, hydrogenated soybean lecithin 150mg, disalmitate lecithin 150mg, put them in a round bottom flask, add 15ml of ethanol to dissolve, 40~50℃ water bath, Rotary evaporation under reduced pressure, evaporate ethanol to form voriconazole mixed micelle film, then add 1600mg lactose and appropriate amount of water for injection to disperse, get voriconazole mixed micelle solution, filter with 0.22 micron microporous membrane, dilute to 10ml, fill in In a vial, press half stopper, freeze-dry, press stopper, press aluminum cap to make voriconazole freeze-dried micelles, each containing voriconazole 200mg. The prepared sample was completely redissolved within 2 minutes, and became a colorless clear liquid with an average particle size of 40nm.
Embodiment 2
[0036] The preparation of embodiment 2 voriconazole lyophilized micellar preparation
[0037]Weigh voriconazole 200mg, sodium glycocholate 500mg, sodium taurodeoxycholate 250mg, hydrogenated soybean lecithin 250mg, disalmitate lecithin 250mg, put them in a round bottom flask, dissolve them in 30ml ethanol, bathe in water at 40-50℃, reduce Press rotary evaporation, evaporate ethanol to form voriconazole mixed micelle film, then add 1050mg dextran and appropriate amount of water for injection to disperse to obtain voriconazole mixed micelle solution, filter with 0.22 micron microporous membrane, dilute to 10ml, fill in cillin In the bottle, press half stopper, freeze-dry, press stopper, press aluminum cap to make voriconazole freeze-dried micelles, each containing voriconazole 200mg. The prepared sample was completely redissolved within 1 minute, and was a colorless clear liquid with an average particle size of 25nm.
Embodiment 3
[0038] The preparation of embodiment 3 voriconazole lyophilized micellar preparation
[0039] Weigh voriconazole 200mg, sodium glycocholate 600mg, sodium taurodeoxycholate 300mg, hydrogenated soybean lecithin 200mg, disalmitate lecithin 200mg, put them in a round bottom flask, add 40ml of ethanol to dissolve, 40~50℃ water bath, Rotary evaporation under reduced pressure, evaporate ethanol, form voriconazole mixed micelle film, add 1000mg mannitol and appropriate amount of water for injection to disperse, get voriconazole mixed micelle solution, filter with 0.22 micron microporous membrane filter, dilute to 10ml, fill in In a vial, press half stopper, freeze-dry, press stopper, press aluminum cap to make voriconazole freeze-dried micelles, each containing voriconazole 200mg. The prepared sample was completely redissolved within 1 minute, and became a colorless clear liquid with an average particle size of 20nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com