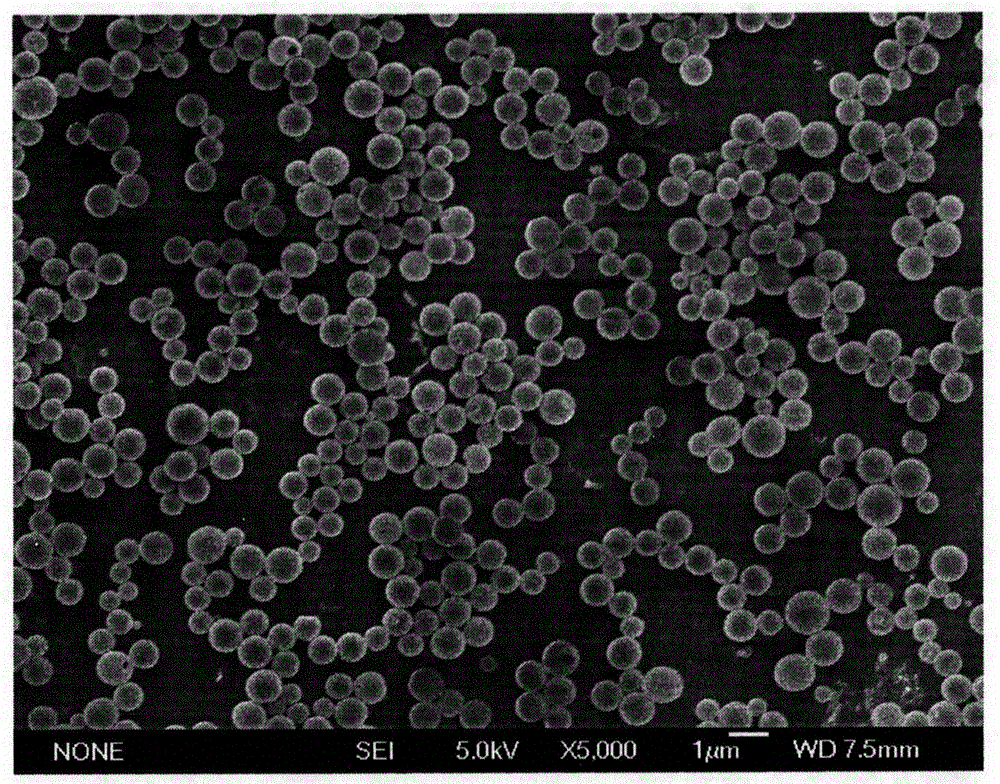
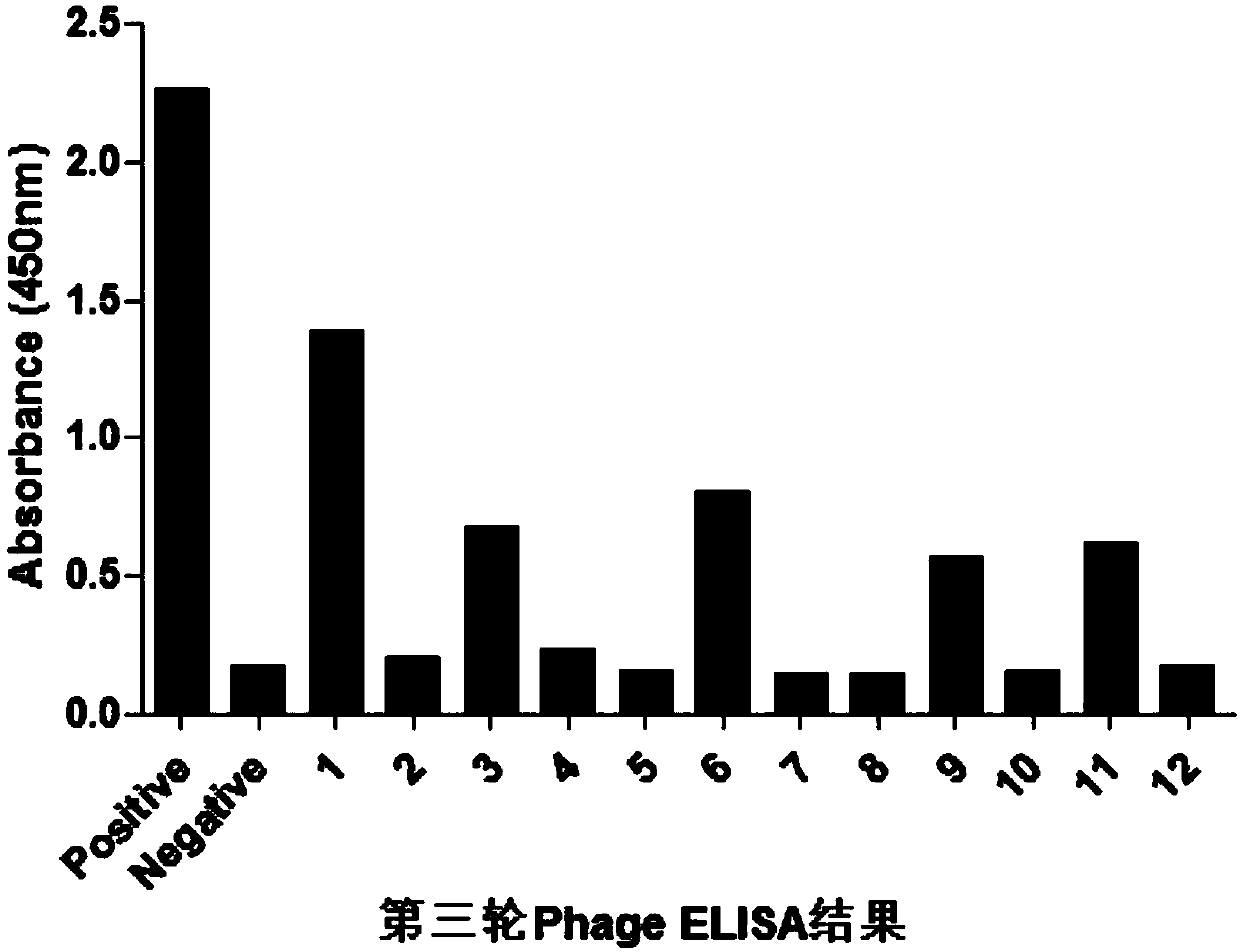
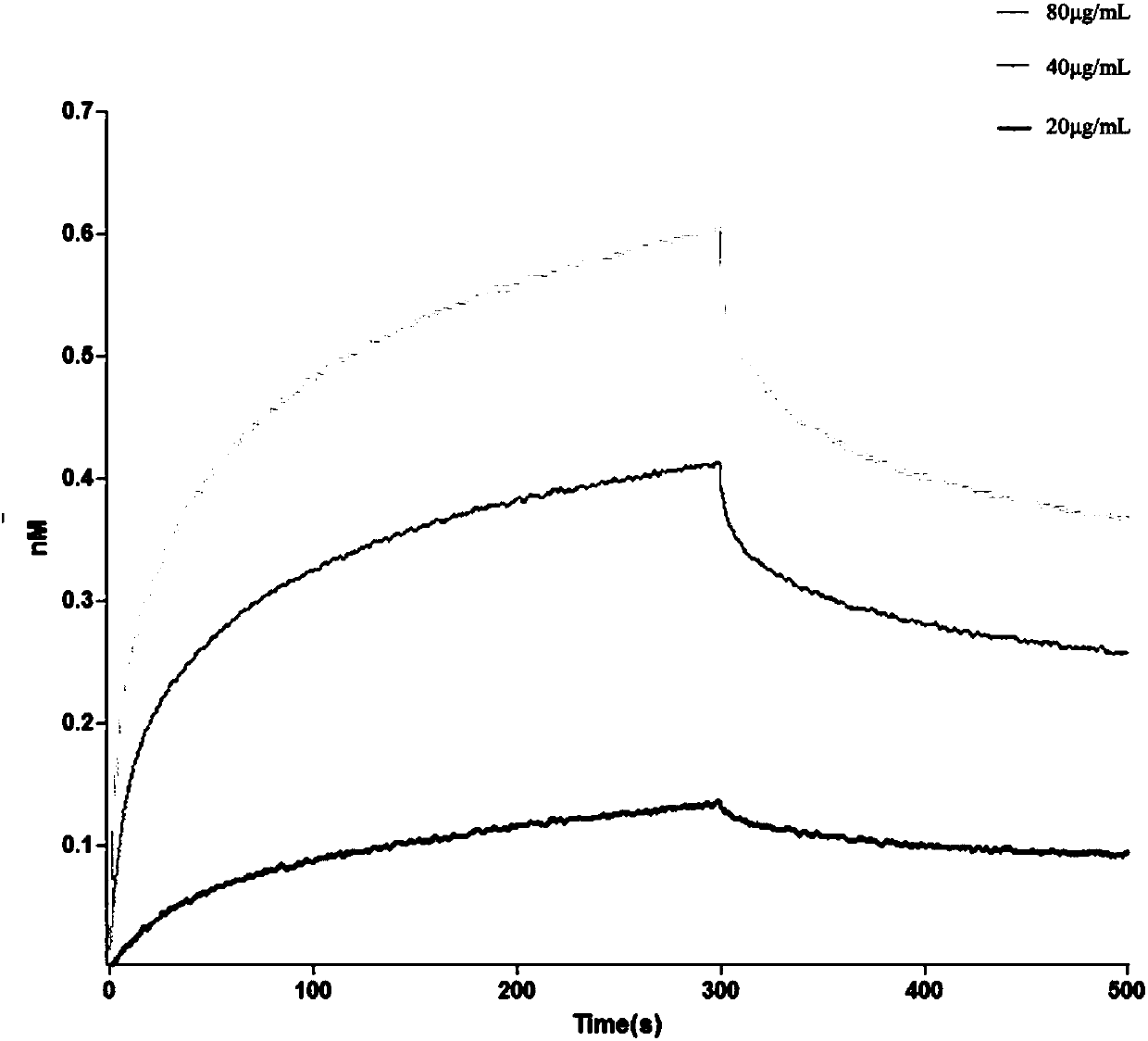
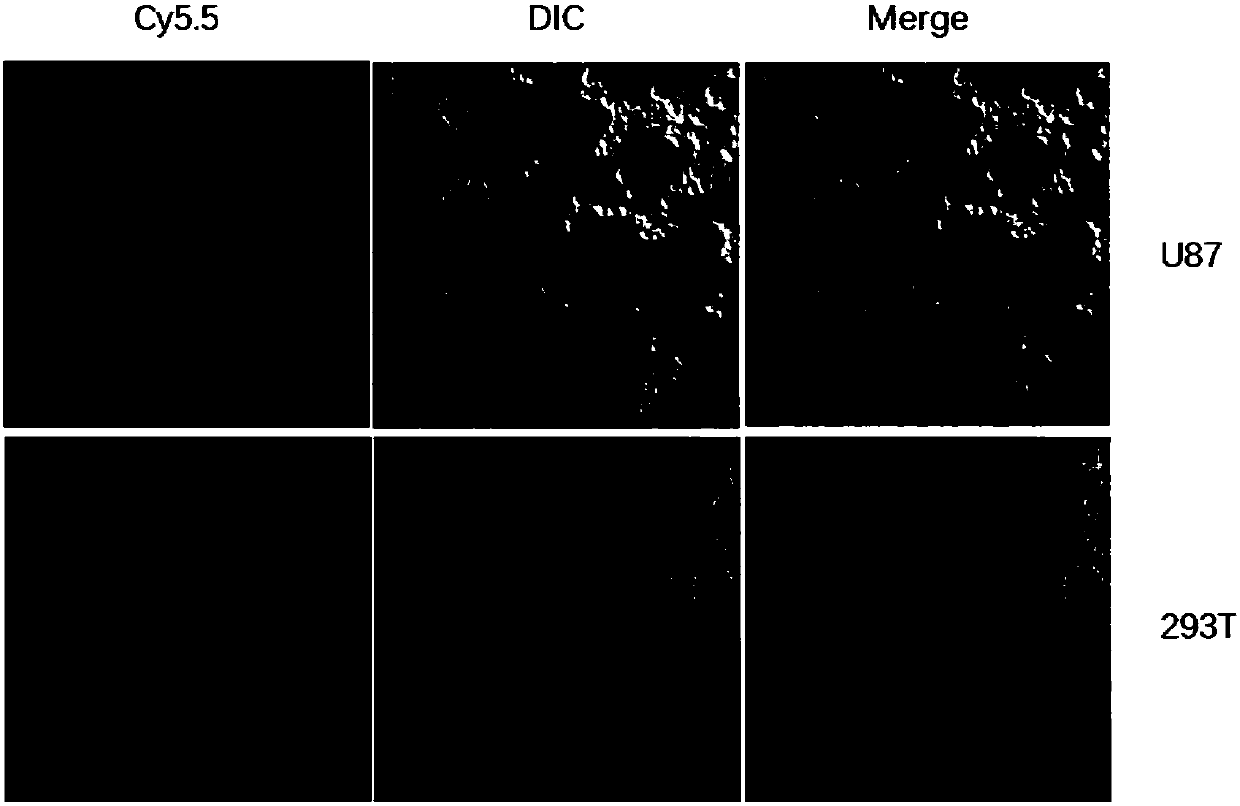
Patents
Literature
148results about How to "Does not cause allergic reactions" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Hydrophilic polyurethane pressure-sensitive adhesive and preparing method thereof
InactiveCN1456630AImprove water absorptionImprove moisture vapor transmission rateFilm/foil adhesivesPolyureas/polyurethane adhesivesPolyurethane adhesivePolyethylene glycol
A pressure-sensitive hydrophilic polyurethane adhesive for medical purpose is prepared from diisocyanate through polycondensating with at least 3 kinds of macro-molecular polyol (polyethanediol or its derivative, hydrophobic macro-molecular biol, and macro-molecular polyol containing at least 3 hydroxy groups) to obtain prepolymer, and enlarging chain via low-molecular diamine or its polymer. Its advantages are good hydrophilicity, and good compatibility to skin.
Owner:TIANJIN UNIV
Spatholobus stem natural dye preparation and its uses
InactiveCN1995151AAbundant raw materialsSimple ingredientsNatural dyesDyeing processTrisodium phosphatePhotochemistry
The invention discloses a preparing method and application of natural dye of spatholobus stem, which comprises the following steps: grinding spatholobus stem; immersing in the sodium carbonate solution with pH value at 8-10 under 95-100 deg. c for 60-90min; filtering to obtain the primary liquid of natural dye; condensing the primary liquid; solidifying; grinding; obtaining the powder-shaped natural dye.
Owner:ZHEJIANG SCI-TECH UNIV +1
Method for preparing polypeptide beverage by using chickpea
InactiveCN102028036ALow antigenicityImprove digestibilityMilk substitutesFood scienceDiabetes mellitusBiological value
The invention discloses a method for preparing polypeptide beverage by using chickpea, which comprises the following steps of: soaking the picked chickpea into warm water, cleaning the soaked chickpea by using clean water, naturally fermenting the chickpea for 3 to 4 days at the temperature of between 22 and 25 DEG C, changing water for 2 to 4 times every day, pulping the fermented chickpea by using a rough pulping machine of 40 to 80 meshes, a fine pulping machine of 80 to 120 meshes and a finished pulping machine of 120 to 160 meshes in turn, then performing enzymolysis by using pawpaw protein enzyme, and homogenizing, sterilizing and bulking the enzymolysis milk. The chickpea protein is subjected to enzymolysis by using biotechnical means to generate polypeptide; the chickpea polypeptide has good low antigenic property, is enough to ensure that the eaten polypeptide food does not cause anaphylactic reaction, and has multiple health-care functions of reducing blood fat and cholesterol, inhibiting diabetes, resisting cancers and the like; and when the chickpea polypeptide is prepared into the easily digested and absorbed beverage, the digestibility and the biological value of the chickpea protein are improved, and the method is suitable for large-scale industrial production.
Owner:陈漫霞
Human mesenchymal stem cell serum-free medium and preparation method thereof
InactiveCN108251360AClear ingredientsThe preparation method is simple and easyCulture processSkeletal/connective tissue cellsSurface markerSerum free media
The present invention relates to a human mesenchymal stem cell serum-free medium, the main components of the human mesenchymal stem cell serum-free medium include a basal medium and an additive; the mesenchymal stem cell serum-free medium has a clear composition and no animal-derived component. A mesenchymal stem cell product produced by the human mesenchymal stem cell serum-free medium is used clinically without causing anaphylactic reaction; and the serum-free medium is simple in preparation method, does not require any special equipment, and is easy to be mass-produced; and mesenchymal stemcells produced by the human mesenchymal stem cell serum-free medium have good cell homogeneity and high proliferation rate. After repeated passages, detection results of cell surface markers are up to standard, and the cells still have good capability of differentiation into osteoblasts, adipocytes and chondrocytes.
Owner:XIAN TISSUE ENG & REGENERATIVE MEDICINE RES INST
Method for synthesizing polysorbate-80 for injection
ActiveCN101983977AHigh purityEfficient removalPharmaceutical non-active ingredientsDistillationEthylene oxide
The invention provides a method for synthesizing polysorbate-80 for injection, comprising the following steps: (1) taking sorbierite as a raw material, partially dehydrating the sorbierite under the conditions of an acid catalyst and a vacuum state, and carrying out crystallization and purification for three times on the dehydrated sorbierite to obtain primary high-purity dehydrated sorbierite II; (2) further dehydrating the high-purity primary dehydrated sorbierite II and then carrying out reduced pressure distillation and purification to obtain secondary high-purity dehydrated sorbierite III; (3) mixing the high-purity primary dehydrated sorbierite II with the high-purity secondary dehydrated sorbierite III in proportion to obtain high-purity dehydrated sorbierite IV; carrying out addition polymerization on the high-purity dehydrated sorbierite IV under the condition of a basic catalyst and ethylene oxide to obtain dehydrated sorbierite polyoxyethylene ether V; and (4) reacting the dehydrated sorbierite polyoxyethylene ether V with high-purity oleic acid under the action of an esterification catalyst and then refining the reactant of the dehydrated sorbierite polyoxyethylene ether V and the high-purity oleic acid to obtain the polysorbate-80 for injection. The liquid chromatogram (LC) of the polysorbate-80 for injection has two principal product peaks without other impurity peaks; and compared with the conventional craft products, the polysorbate-80 for injection is higher in purity, more stable in the technology, is controllable, meets the injection requirements in quality and has more reliable safety in clinical application.
Owner:南京威尔药业科技有限公司
Preparation method of rubber and plastic synthetic ultrathin condom
ActiveCN104479182AReduce the impactImprove performanceMale contraceptivesCoatingsWater basedPolymer science
The invention discloses a preparation method of a rubber and plastic synthetic ultrathin condom. The preparation method comprises the following steps: by taking synthesized polyisoprene as a matrix, adding the synthesized polyisoprene, water-based polyurethane, natural rubber latex, KOH, ammonia water and an initiator under a certain condition, thereby synthesizing the condom prepared from a composite high polymer material. According to the preparation method of the rubber and plastic synthetic ultrathin condom disclosed by the invention, the preparation method adopting the synthesized polyisoprene, a little natural rubber latex and water-based polyurethane is used to substitute the original natural rubber latex process to prepare a condom product. The condom is not added with vulcanizing agents sulfur, zinc oxide, an accelerator, and the like, is slightly influenced by environment, and is increased in product qualified rate; the raw materials are good in performance and can be used for preparing the ultrathin condom which has the advantages of good skin affinity, no water-soluble proteins, no anaphylactic reaction on the human body, and the like.
Owner:SUZHOU COLOR WAY ENTERPRISE DEV
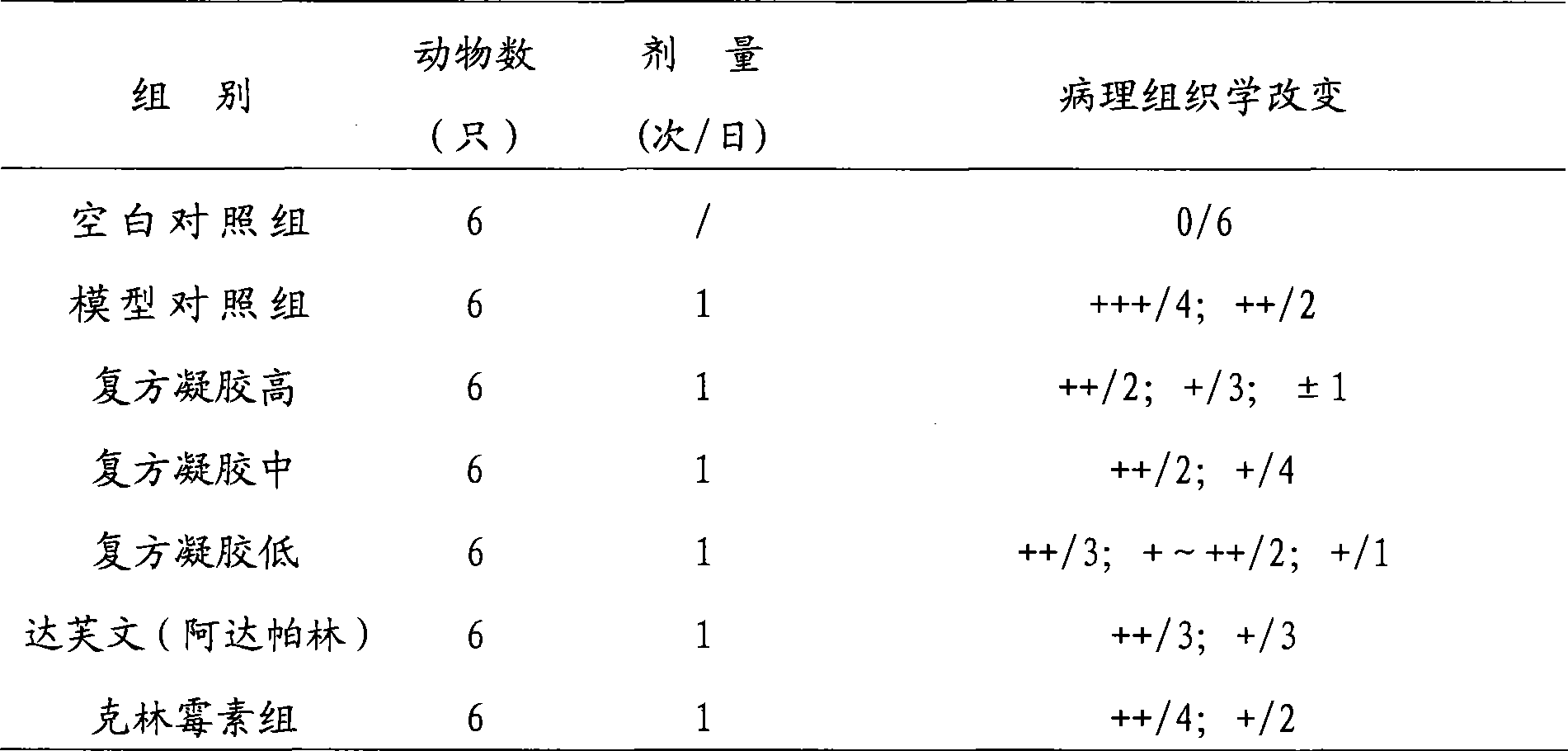
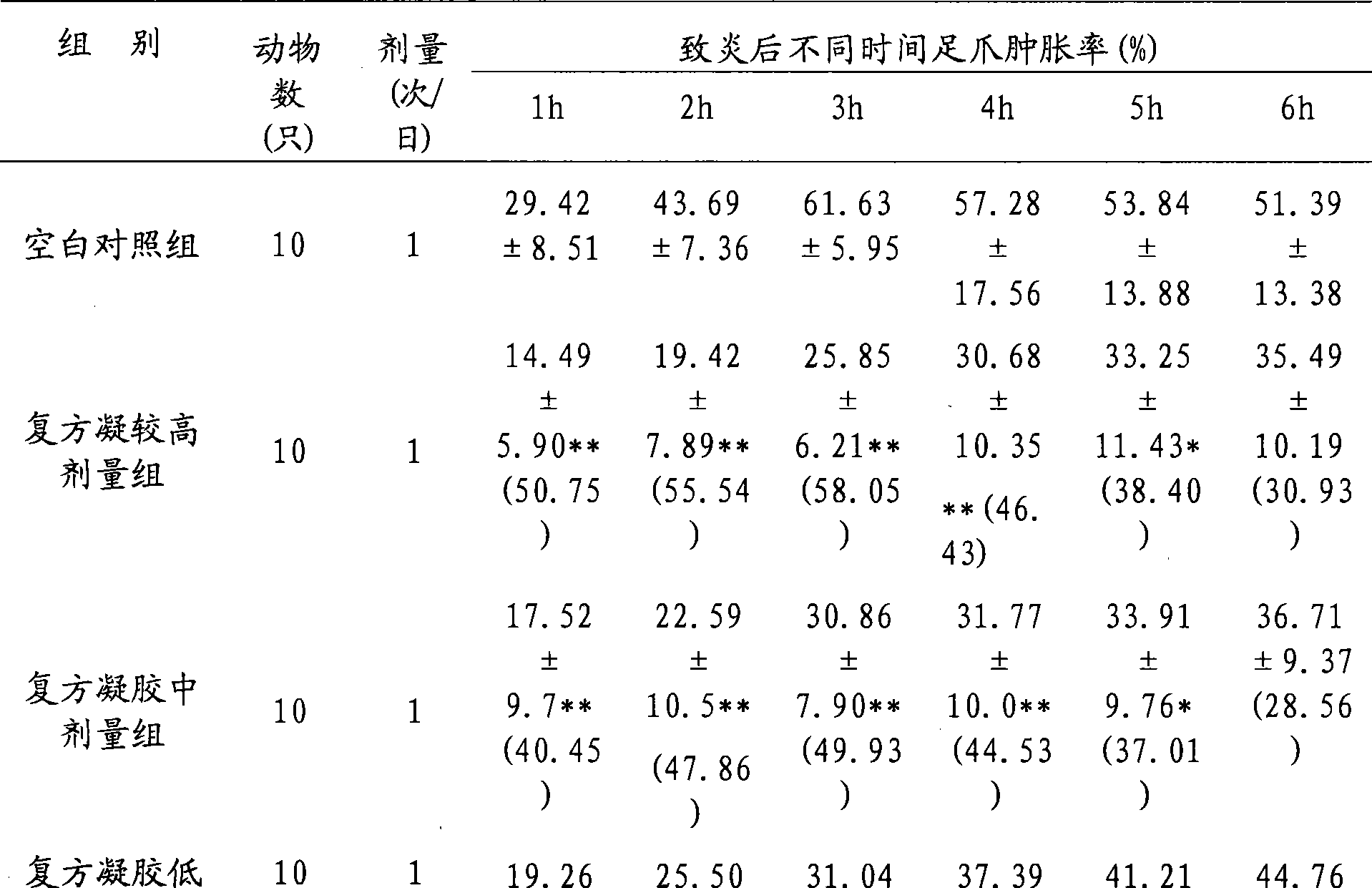
Adapalene and hydrochloric clindamycin compound gel preparation and preparation method thereof
ActiveCN101485675AEffective treatmentThe therapeutic effect of the preparation is remarkableOrganic active ingredientsPharmaceutical delivery mechanismClindamycin HydrochlorideGel matrix
The invention discloses an adapalene clindamycin hydrochloride compound gel preparation and a preparation method thereof. The preparation contains 0.08 to 0.12 percent of adapalene and 0.8 to 1.2 percent of clindamycin hydrochloride according to the weight percentage. The preparation method for the adapalene clindamycin hydrochloride compound gel comprises the steps of: preparing a gel matrix, swelling, sterilizing, dissolving the adapalene and the clindamycin hydrochloride into the gel matrix respectively, and using triethanolamine to adjust the pH value to form the steady gel. The product can effectively treat acne.
Owner:ZHAOKE GUANGZHOU OPTHALMIC DRUG
Slow-release and anti-infective composite soft tissue repair material and preparation method thereof
The invention relates to a slow-release and anti-infective composite soft tissue repair material and a preparation method thereof. The repair material is prepared from a biological material and media containing anti-infective components, wherein the media containing the anti-infective components are arranged between layers of the biological material or on the surface of the biological material at intervals according to the radius of an inhibition zone of a drug sensitive test, and a whole body is formed by the aid of a binding agent, suture lines or pressure. According to the slow-release and anti-infective composite soft tissue repair material and the preparation method thereof, the anti-infective components can be released effectively and continuously, meanwhile, biocompatibility of the biological material is not changed, the biological material is non-toxic and harmless, and the application prospect is good.
Owner:EXCELLENCE MEDICAL TECH SUZHOU CO LTD +1
Skin lotion with moisturizing, anti-aging and whitening and freckle removing functions
InactiveCN109044929AStrong moisturizingStrong anti agingCosmetic preparationsToilet preparationsIrritationHypersensitive response
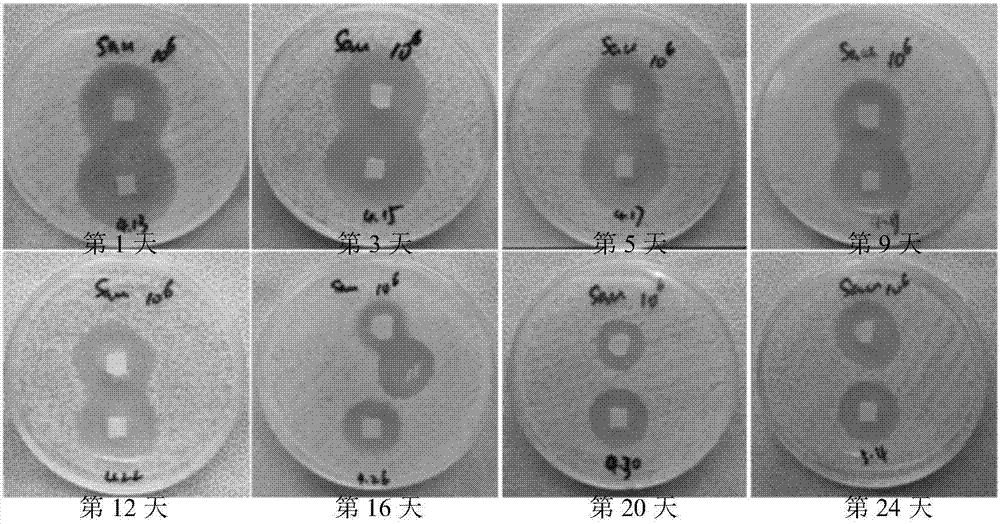
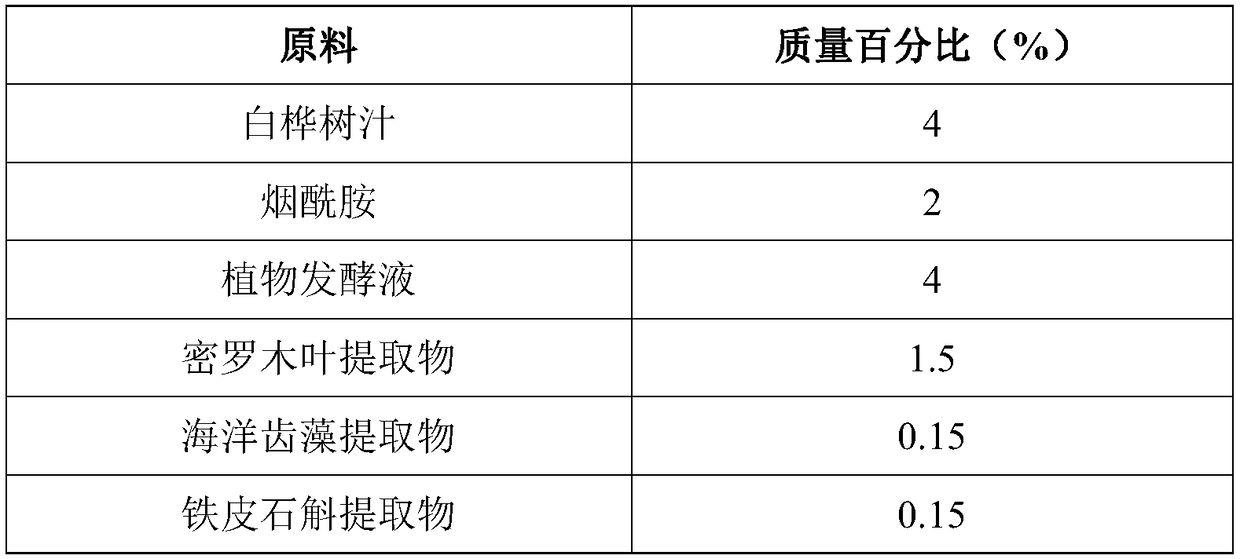
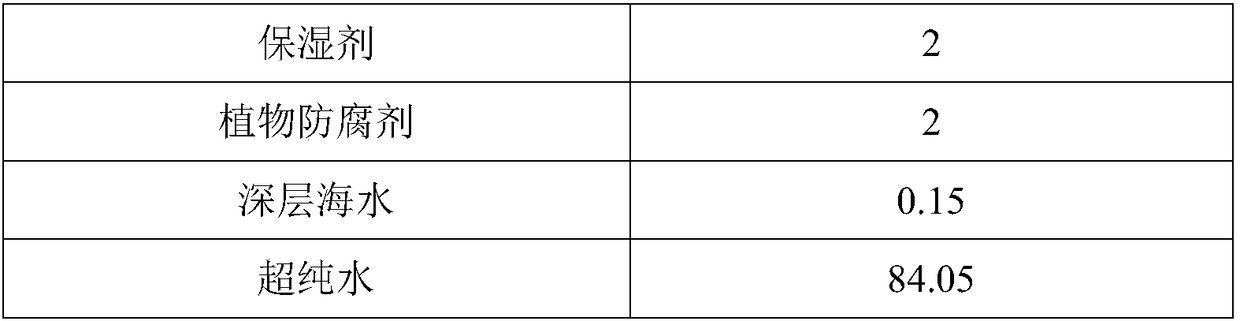
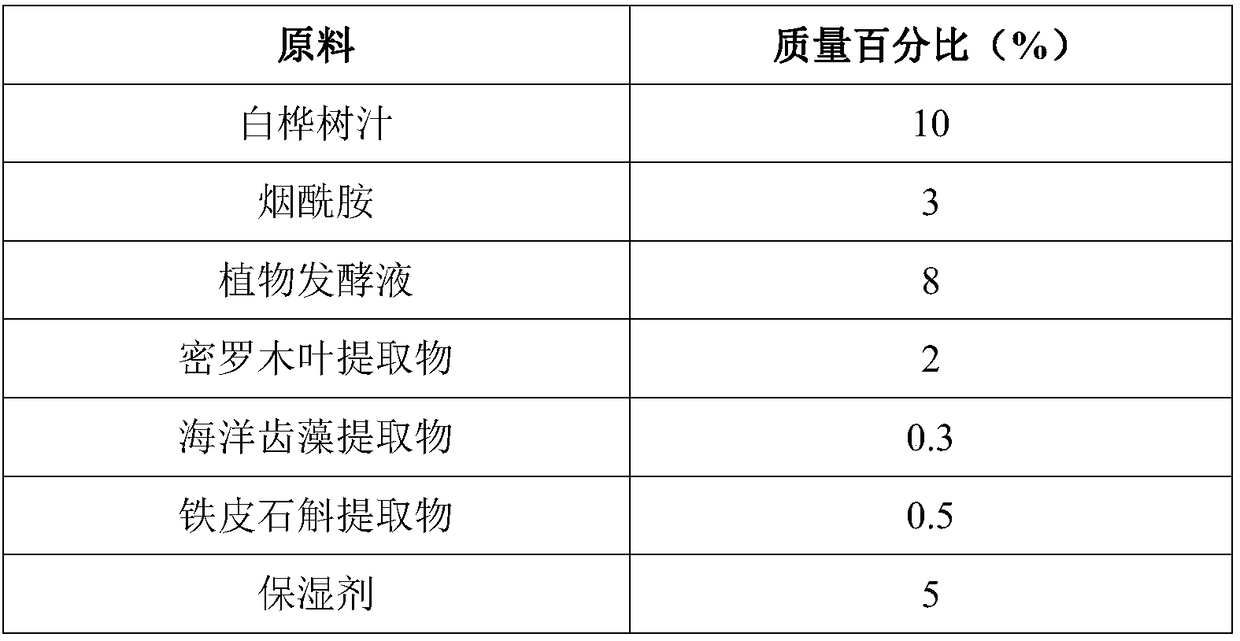
The invention discloses a skin lotion with moisturizing, anti-aging and whitening and freckle removing functions. The skin lotion is prepared from white birch juice, nicotinamide, plant fermentation broth, a myrothamnus flabellifolia leaf extract, a marine dentiform algae extract, a dendrobium officinale extract, a humectant, a plant preservative, deep sea water and water. A preparation method ofthe skin lotion is very simple, and the raw materials are uniformly mixed in batches. The skin lotion disclosed by the invention has strong moisturizing, anti-aging, whitening and freckle removing effects, can significantly improve smoothness, brightness, tightness and anti-irritation ability of the skin, is non-toxic to the skin and mucous membrane, does not cause allergy, and is pleasant in fragrance and high in stability.
Owner:陶萍
Mushroom hand cream and preparation method thereof
InactiveCN105030624AIncrease elasticityPrevent wrinklesCosmetic preparationsToilet preparationsCetostearyl alcoholGlycerol
The invention belongs to the technical field of daily skincare products and particularly relates to mushroom hand cream and a preparation method thereof. The mushroom hand cream is composed of, by weight, 10-20 parts of needle mushroom, 5-15 parts of hericium erinaceus, 10-15 parts of pleurotus ostreatus, 12-16 parts of grifron maitake, 1-3 parts of mint, 1-3 parts of osmanthus fragrans essential oil, 2-6 parts of glycerinum, 2-4 parts of sodium lauryl sulfate and 1-3 parts of cetostearyl alcohol. The preparation method is simple, the obtained hand cream has moisturizing and beautifying effects, the anti-allergic and anti-aging healthcare effects and the like, and the mushroom hand cream is safe for the human body and free of side effects on the human body.
Owner:福建绿宝食品集团有限公司
Transdermal Ligustrazine plaster and its prepn
InactiveCN1432363AReduce defectsStable blood concentrationOrganic active ingredientsMedical devicesTransdermal patchSide effect
The present invention belongs to the field of pharmaceutical technology and relates to transdermal Ligustrazine plaster and its preparation. It has Ligustrazine phosphate or Ligustrazine as active medicinal component and consists of medicine storing back, adhering layer, release controlling film, back lining layer and protective layer. It is superior to available Ligustrazine preparation forms inmedicine releasing are, transdermal rate and lasting release time, and has the features of determined curative effect, stable quality, high safety, etc. Animal experiments show that the plaster can retard the effect of ischemic to cerebral damage, inhibit thrombosis caused by tissue damage, prevent and treat cerebral embolism and maintain balanced medicine density in blood for 24 hr; and has light irritation to skin, no irritability and no toxic reaction.
Owner:FUDAN UNIV +1
Medical mesh for hernia repair patch
The invention relates to a medical mesh for a hernia repair patch. The mesh is characterized by adopting medical grade polycaprolactone monofils as weaving material and having a breaking strength of 500 to 700 CN, a breaking elongation of 30 to 100 percent, a knot breaking strength of 400 to 600 CN and a knot breaking elongation of 10 to 50 percent. Besides the basic physical and chemical properties of common polycaprolactone, the medical grade polycaprolactone surpasses the common polycaprolactone regarding the purity, biological stability and biological solubility because the components of parent material and low molecular additives are strictly controlled in the processing process. The polycaprolactone monofils has a higher rigidity and is more suitable for being used to weave the hernia repair patch which has certain strength and bending rigidity and is capable of resisting human body abdominal pressure and defected body pressure.
Owner:DONGHUA UNIV
Baby body wash and preparation method thereof
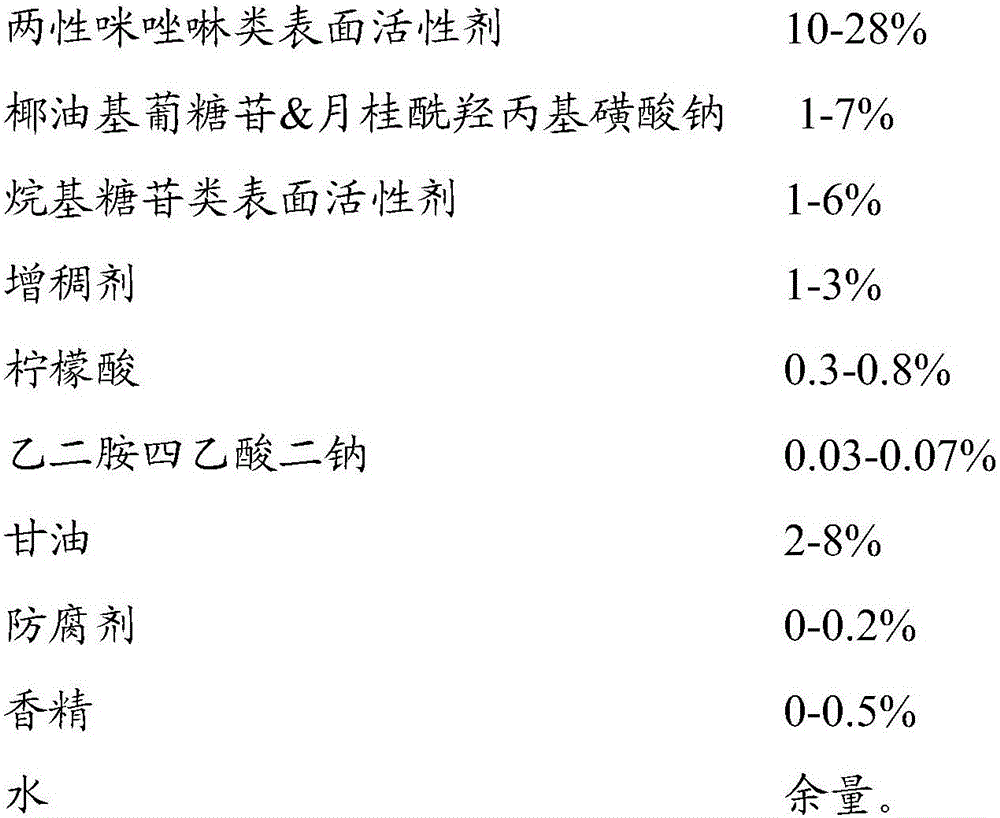
InactiveCN106137789AProperly cleanedNon-irritatingCosmetic preparationsToilet preparationsAllergic reactionBalance water
The invention relates to baby body wash and a preparation method thereof. The baby body wash is prepared from, by weight, 5-30% of amphoteric imidazoline surfactant, 1-10% of coco-glucoside and sodium lauroyl hydroxypropyl sulfonate, 1-10% of alkyl glycoside surfactant, 0.1-5.0% of a thickening agent, 0.2-1.0% of citric acid, 0.01-0.08% of ethylenediamine tetraacetic acid, 1-10% of glycerin, 0-0.3% of preservatives, 0-0.5% of essences and the balance water. The baby body wash is quite mild, completely accords with baby skin characteristics and is free of skin irritation and allergic reaction while proper cleaning of baby skin is guaranteed; due to pH of the baby body wash is 5.5-6.5, the baby body wash can better accord with intrinsic pH characteristics of baby skin, maintain a natural barrier function of baby skin and better retain moisture.
Owner:GUANGZHOU HONDU FINE CHEM CO LTD
Freeze-dried voriconazole micelle preparation and preparation method thereof
InactiveCN102335118AGood biocompatibilityImprove securityOrganic active ingredientsAntimycoticsSolubilityMixed micelle
The invention discloses a freeze-dried voriconazole micelle preparation and a preparation method thereof. The freeze-dried voriconazole micelle preparation consists of the following components in part by weight: 1 part of voriconazole, 2 to 10 parts of cholate, 1.5 to 5 parts of phospholipid, and 1 to 10 parts of freeze-drying supporting agent. According to the preparation, the dissolubility of the voriconazole is increased, and the dissolution degree of the voriconazole in water is improved; and the preparation can be diluted into intravenous injection by using injection water for clinical use. The particle diameter of the prepared voriconazole micelles is between 10 and 100 nanometers, the particle diameter of most micelles is about 20 nanometers, the particle size is uniform, the frozen and dried voriconazole micelles are high in re-dissolution speed, a transparent and colorless solution is formed after the voriconazole micelles are re-dissolved, and the particle diameter has no obvious change, so the safety of clinical application is enhanced. According to the prepared freeze-dried voriconazole micelle preparation, because the voriconazole is wrapped inside by the mixed micelles, influence of light, heat and moisture is avoided, and the stability of the voriconazole in a long-term storage process is improved.
Owner:JINAN KANGHE MEDICAL TECH
Transgenic cow obtaining method
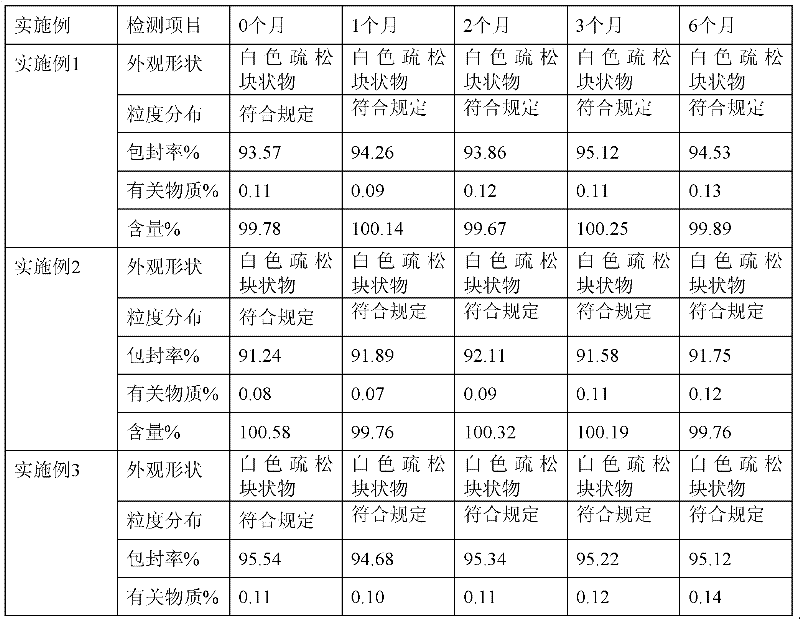
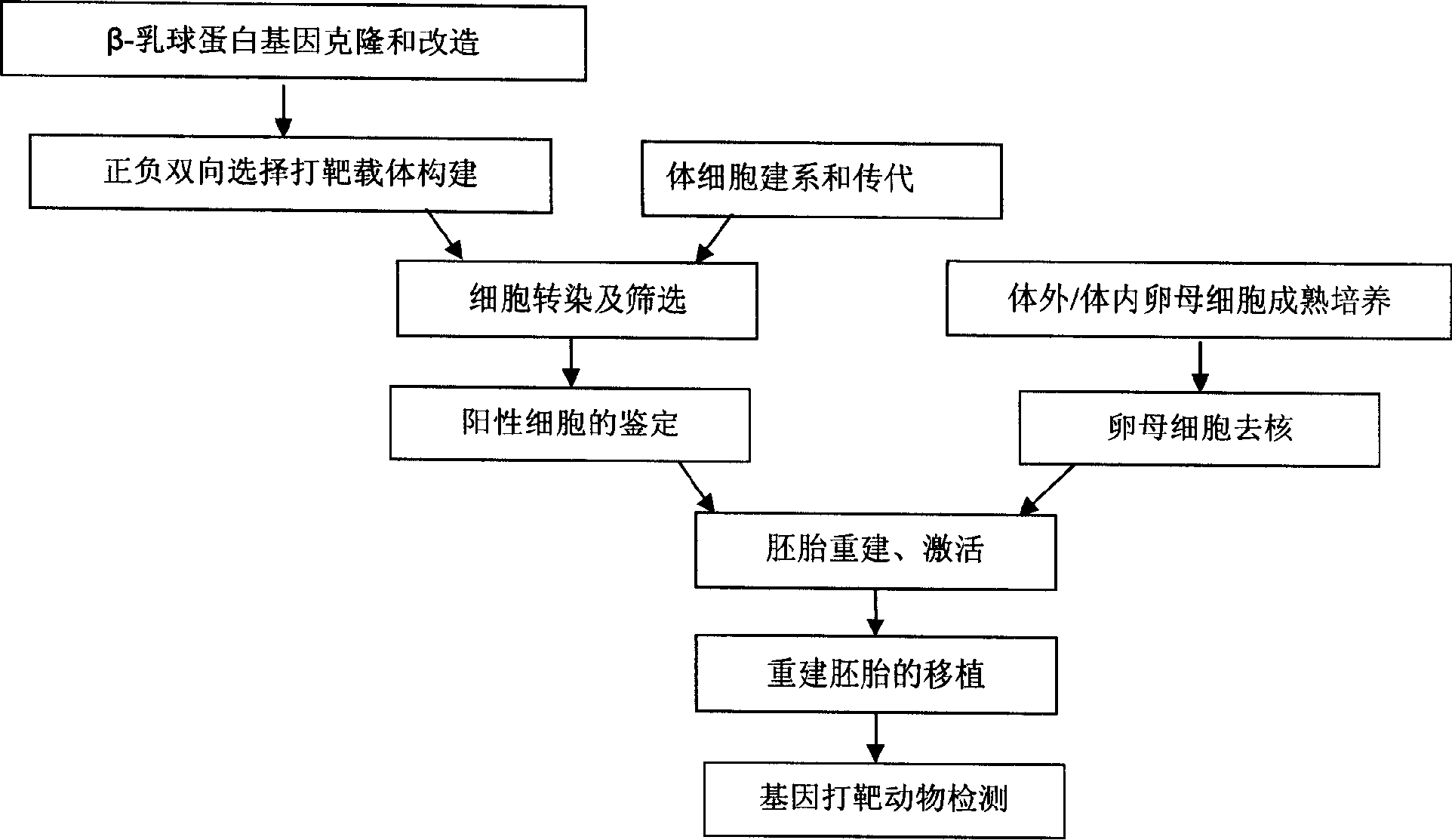
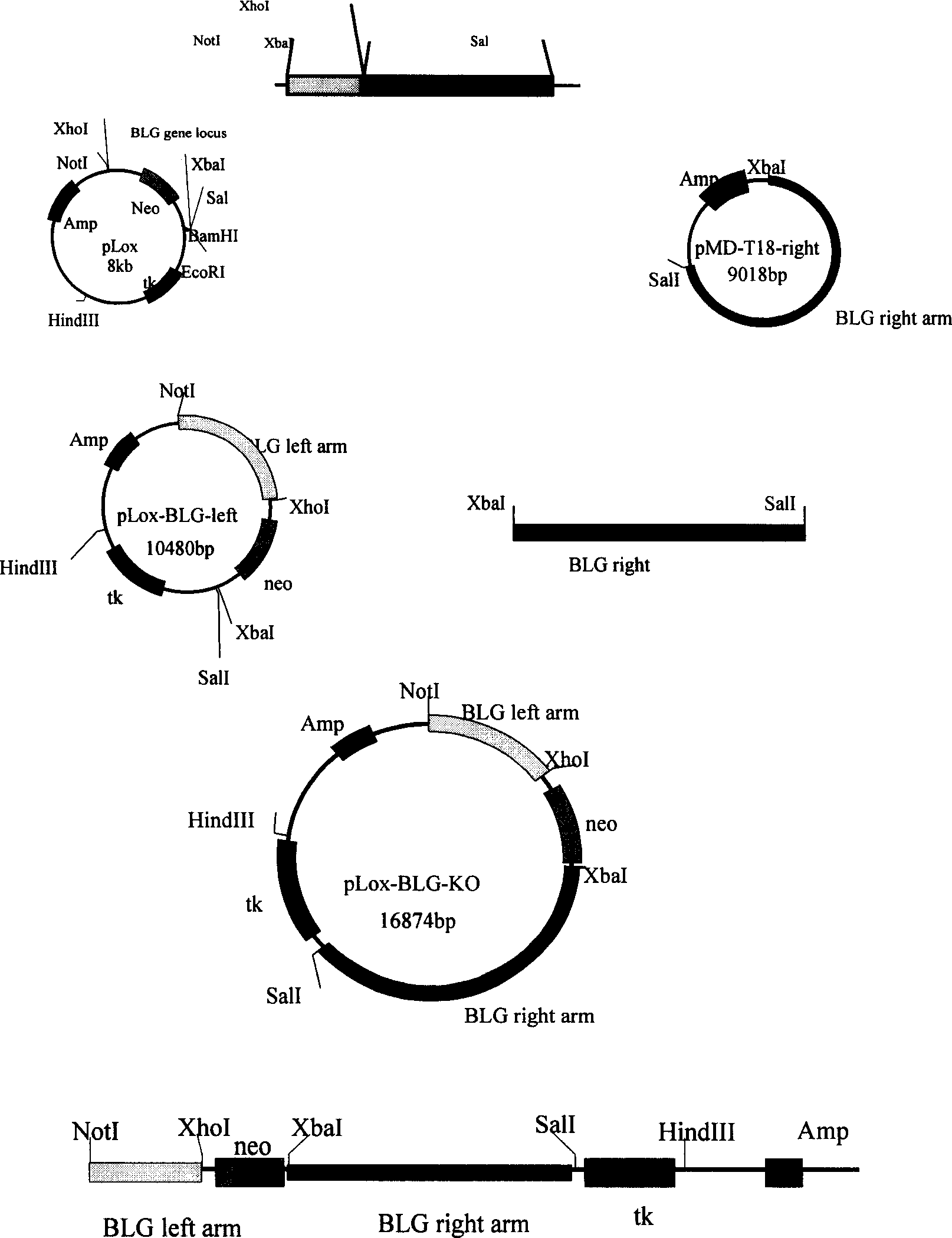
InactiveCN1865448ADoes not cause allergic reactionsEat a wide range of peopleFermentationVector-based foreign material introductionHypersensitive responseEmbryo
The invention discloses an obtaining method of gene-transition cow, which comprises the following steps: connecting target carrier with linear beta-milk globulin protein mutation gene with somatic cell through isogenesis recombination; forming beta-milk globulin protein gene silence conduct cell; guiding the conduct cell in the cow enucleated mother cell to rebuild embryo; transplanting the recombined embryo in the pregnant cow uterus. The method makes cow produce more milk without beta-milk globulin protein, which is more nutritious for people.
Owner:李宁
Novel adjuvant vaccine composition associated with HPV
The present invention discloses a novel adjuvant vaccine associated with HPV, and a preparation method thereof, wherein specifically the novel adjuvant is PLGA-CaP composite particles, and the adjuvant vaccine PLGA-CaP composite particles can produce in vivo immune response, and belongs to the developed efficient HPV protein vaccine composition.
Owner:张世艳 +1
Pharmaceutical composition comprising lipid-soluble vitamin for injection and preparation method of pharmaceutical composition
ActiveCN103520186AImprove complianceIncrease fat solubilityPowder deliveryMetabolism disorderSolubilityFreeze-drying
The invention relates to a pharmaceutical composition comprising lipid-soluble vitamin for injection, which improves water solubility, and a preparation method of the pharmaceutical composition. The pharmaceutical composition comprises vitamin A palmitate, vitamin D2, vitamin E, vitamin K1, water-soluble carboxylation chitosan and mannite. The preparation method comprises the steps of medicine liquid preparation, activated carbon adsorption, decarburization, filtering sterilization, filling, freeze-drying and the like. The pharmaceutical composition comprising lipid-soluble vitamin for injection utilizes the water-soluble carboxylation chitosan as a solubilizer, and has the advantages of few accessories, good water solubility, stable and controllable quality, high safety, simple preparation method, easiness in industrialization and the like.
Owner:HAISCO PHARMA GRP INC
EDB-FN protein targeting peptide with high affinity and application thereof
ActiveCN107903307ADoes not cause purgeNot easy to removeBiological material analysisPeptidesProtein targetPerformed Diagnosis
The invention relates to a fibronectin EDB subtype specific targeting short peptide A16 with an amino acid sequence of ATYRLFQGVEVV. The targeting short peptide A16 can specific recognize EDB-FN protein expressed by tumor cells, has high affinity to EDB-FN and is capable of carrying functional molecules with diagnosis or therapeutic activity to tumor cells to perform diagnosis or treatment effect.
Owner:PEKING UNIV
Walnut meal emulsion with effects of freckle fading, whitening, moisturizing and skin tendering, and production method thereof
InactiveCN105055198ASuppress generationReduce synthesisCosmetic preparationsToilet preparationsGlycerolPyrrolidinones
The present invention discloses a walnut meal emulsion with effects of freckle fading, whitening, moisturizing and skin tendering, and a production method thereof, wherein the raw materials comprise, by weight, 3-6 parts of a walnut meal extract, 0.2-0.5 part of sodium hyaluronate, 6-10 parts of glycerol, 4-8 parts of sodium pyrrolidone carboxylate, 6-12 parts of 1,2-propanediol, 0.5-0.8 part of vitamin E, 0.4-0.7 part of silk peptide powder, 3-5 parts of glyceryl monostearate, 0.8-1.2 parts of lecithin, 0.1-0.4 part of methylparaben, 0.1-0.4 part of diazolidinyl urea, 0.02-0.06 part of essence, and 56.94-72.88 parts of deionized water. According to the present invention, the walnut meal extract is added, such that the tyrosinase activity can be inhibited so as to effectively reduce the melanin generation; the walnut meal extract can effectively eliminate free radicals; and the walnut meal emulsion has effects of rapid chloasma fading, good moisturizing, good anti-oxidation effect, no irritation on skin, and no toxic-side effect.
Owner:KUNMING UNIV OF SCI & TECH
Make-up base cream containing cyperus esculentus root oil and preparation method of make-up base cream
InactiveCN111317673AAvoid damageEnhanced barrier functionCosmetic preparationsToilet preparationsBase creamDry skin
The present invention discloses a make-up base cream containing cyperus esculentus root oil and a preparation method of the make-up base cream. Cyperus esculentus root oil rich in vitamin E and linoleic acid is combined with whitening, moisturizing and anti-aging ingredients, a modern technology is used to obtain the make-up base cream containing the cyperus esculentus root oil, and the make-up base cream has stable performance, has effects of isolation, whitening, concealing, skin tone adjusting and sunscreen, also has skin-care functions of moisturizing skin, improving skin gloss and tenderizing the skin, repairs damaged skin barrier, alleviates conditions of skin dryness, roughness and aging, and fundamentally solves skin problems.
Owner:宋超
Moisturizing anti-wrinkle facial mask and preparation method thereof
InactiveCN108245466AImprove antibacterialGood antibacterial effectCosmetic preparationsToilet preparationsPreservativeMedicine
The invention relates to a moisturizing anti-wrinkle facial mask and a preparation method thereof. The moisturizing anti-wrinkle facial mask is prepared from the following components of an emulsifyingagent, a moisturizing agent, a preservative, skin moisten grease, a PH adjusting agent, anti-wrinkle restore matter and the balance of distilled water. The anti-wrinkle restore matter is prepared from the following ingredients of a Chrysanthemum bud extract, an aloe pectin extract, a calendula flower extract and a lemon extract. The preparation method comprises the following steps of S1 preparation of the anti-wrinkle restore matter, S11 preparation of the chrysanthemum bud extract, S12 preparation of the aloe pectin extraction, S13 preparation of the calendula flower extract, S14 preparationof the lemon extract and S2 preparation of the moisturizing anti-wrinkle facial mask. The moisturizing anti-wrinkle facial mask has the excellent effects of moisturizing, water retaining, allergy releasing, oxidation resistance and stain fading and repairing.
Owner:华巧波
Natural essence capable of resisting wrinkles and tightening skin
InactiveCN107412034AInhibit or eliminate oxidative damageTo promote metabolismCosmetic preparationsToilet preparationsCynomorium songaricumIrritation
The invention discloses natural essence capable of resisting wrinkles and tightening the skin. The natural essence capable of resisting wrinkles and tightening the skin disclosed by the invention contains a plant composition formed by mixing myrtle extract, cynomorium songaricum extract and sweet apricot kernel extract at the weight part ratio of (2-8): (1-4): (2-8). The natural essence capable of resisting wrinkles and tightening the skin disclosed by the invention has the functions of scavenging free radicals, promoting the elasticity of the skin, enabling the skin to be compact and smooth, well nourishing the skin from the deep layer, tendering the skin, and resisting ageing, is pollution-free and safe, is mild without irritation, and can not cause the allergic reaction.
Owner:聚吉减肥塑形科学技术研究院(广州)有限公司
Attapulgite shaving cream
InactiveCN101401772ABroad-spectrum antibacterialUse low concentrationCosmetic preparationsHair removalSulfonateShaving cream
The invention discloses an attapulgite shaving cream, which adopts the technical proposal that the attapulgite shaving cream consists of modified cream attapulgite clay, stearate, coconut oil acyl ethoxyl sodium sulfonate, sodium dodecyl sulfate, citric acid, mint camphor, nipagin methyl ester, nipagin propyl ester and deionized water. A preparation method for the attapulgite shaving cream comprises the following steps: making slurry, treating with sodium, blending, pulping, grinding, stirring at high speed, emulsifying and canning to produce the finished product of the attapulgite shaving cream. The modified cream attapulgite clay can attach to the surface of the skin well, increases the contact area with the beard, is not easy to dry, has no stimulus, does not cause allergic reaction of the skin, and improves the use effect of the shaving cream. The attapulgite shaving cream has the advantages that the attapulgite shaving cream has good hand feeling, mild performance and rich foam, can soften the beard, clean the skin and smooth the skin, and is suitable for shaving beard and fine hair.
Owner:江苏佰腾科技有限公司
Skin penetrating paste containing ligustrazine free alkali and its preparation
InactiveCN1509717AContinuous and stable releaseReduce defectsOrganic active ingredientsMedical devicesTransdermal patchSide effect
A percutaneous picking of liquestrazine free alkali for preventing thrombocyte coagulation, expanding arteriole and improving microcirculation is composed of storage layer, adhesive layer, release controlling film, substrate and protecting layer. Its advantages are stable concentration of medicine in blood, sure curative effect and low by-effect.
Owner:FUDAN UNIV +1
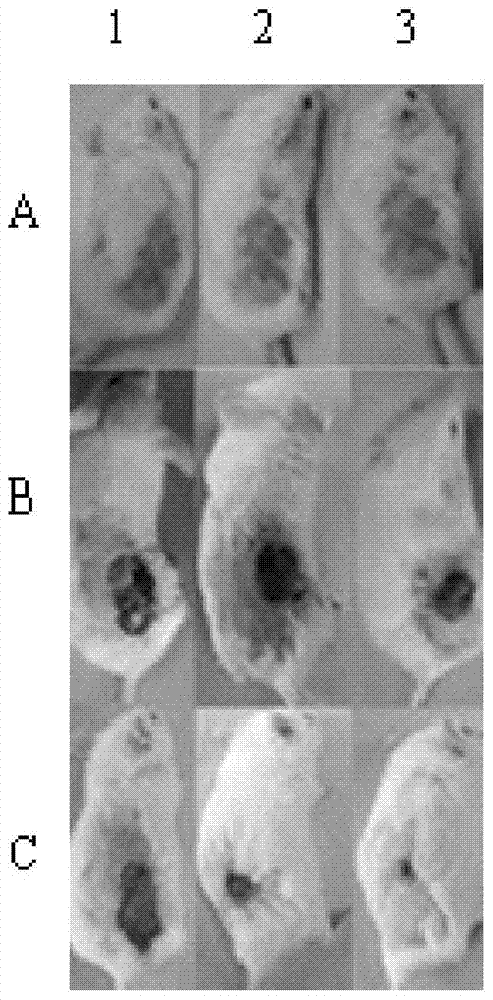
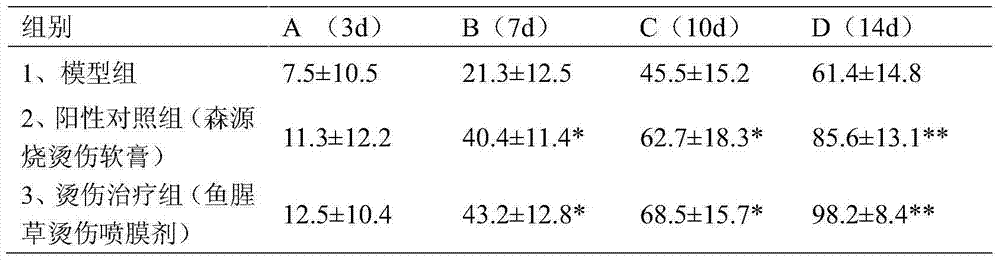
Houttuynia cordata scalding spray film agent and preparation method thereof
InactiveCN103494932AGood treatment effectImprove stabilityHydroxy compound active ingredientsDermatological disorderAlcoholEffective treatment
The invention discloses a houttuynia cordata scalding spray film agent and a preparation method thereof. The houttuynia cordata scalding spray film agent is characterized by comprising, by weight, 75-100 parts of houttuynia cordata mixing leach liquid, 3-5 parts of borneol and 50 parts of polyvinylpyrrolidone. The preparation method of the houttuynia cordata scalding spray film agent is characterized by including the following processing steps that (1) leach liquid is prepared; (2) 50-part polyvinylpyrrolidone is weighed, the polyvinylpyrrolidone is added into the houttuynia cordata mixing leach liquid, and even stirring is carried out; (3) after the borneol with the parts by weight being 3-5 parts is dissolved by ethyl alcohol, the mixed liquid is added into the solution obtained in the step (2), even mixing is conducted, and the houttuynia cordata scalding spray film agent is obtained. According to the houttuynia cordata scalding spray film agent and the preparation method thereof, the method is simple and convenient, the houttuynia cordata scalding spray film agent obtained through preparation can be used for treatment of burning and scalding quickly and effectively, skin allergy is avoided, toxic reaction can not occur to skin, stability is good, and production cost is low.
Owner:CHONGQING NORMAL UNIVERSITY
Non-sensitized self-adhering bandage and preparation method thereof
InactiveCN101406709ADoes not cause allergic reactionsReduce economic costsLiquid surface applicatorsAbsorbent padsSide effectADHESIVE ELASTIC BANDAGE
The invention discloses a non-sensitized self-adhesive elastic bandage and a preparation method thereof, which belong to the technical field of medical material. The non-sensitized self-adhesive elastic bandage comprises a bandage substrate and non-sensitized glue, wherein the non-sensitized glue comprises the following compositions in portion by mass: 100 portions of low-protein or deproteinization natural emulsion, 100 to 150 portions of water, 10 to 50 portions of an emulsifier, 1 to 10 portions of an anti-aging agent, 1 to 10 portions of an antiseptic and 1 to 10 portions of a smoothing agent. The method combines the formula and the preparation technology to prepare the non-sensitized self-adhesive elastic bandage after specially treating the low-protein or deproteinization natural emulsion. The bandage has the advantages that the bandage completely accords with the standards after undergoing biological performance detection, can not cause anaphylaxis of the human skin, is safe to use and has no toxic and side effects; and simultaneously the bandage has moderate self viscosity, only generates self-adhesion, does not adhere to the skin, the hair and so on, and is convenient to use.
Owner:鲁建国
Hair conditioner having hair restoration function and preparation method thereof
InactiveCN104523540APrevent split endsAdd luster to hairCosmetic preparationsHair cosmeticsNutrientChemistry
The invention provides a hair conditioner having hair restoration function and a preparation method thereof. The hair conditioner comprises the following components by weight percentage: 0.2-2% of nut oil, 0.3-3% of olive oil, 0.05-5% of Vaseline, 0.25-2.5% of glyceryl monostearate, 0.01-1% of cetanol, 0.4-4% of isopropyl myristate, 0.21-2% of Freon, 0.45-3% of ethyl acrylate copolymer, 0.2-2.5% of horse oil, 0.1-0.5% of phosphatidylcholine, 0.5-5% of glycerin and balance of deionized water. The hair conditioner contains abundant nutrition components, through compatibility with a surfactant, the effective components have large permeability, the hair conditioner can penetrate in minimal gaps and close the damaged hair squama, nutrient can be rapidly adsorbed by hair, oily feel can not be generated, the hair is healthy and moist, and the hair conditioner can promote self-healing capability and metabolism of hair.
Owner:AESTHETIC TECH BEIJING
Whitening and spot-fading mask and preparation method thereof
InactiveCN108245476AExcellent moisturizing performanceImprove water locking performanceCosmetic preparationsToilet preparationsChemistryWater soluble
The invention relates to a whitening and spot-fading mask and a preparation method thereof. The whitening and spot-fading mask comprises a high-concentration cosmetic liquid, mask paper, a mask papersticker and a packaging bag, wherein the high-concentration cosmetic liquid comprises the following components: a gel, a humectant, a preservative, emollient grease, a pH regulator, whitening and spot-fading substances, and the balance of distilled water; the whitening and spot-fading substances comprise the following components: pearl powder, vitamin E, water-soluble beta-glucan, protein powder,saffron and lutein. The preparation method comprises the following steps: S1, pulverizing and preparing raw materials; S2, preparing aqueous phase components; S3, preparing oil phase components; S4, preparing the high-concentration cosmetic liquid; S5, preparing a mask carrier; S6, preparing the whitening and spot-fading mask. The whitening and spot-fading mask provided by the invention has the advantages of good moisturizing effect, water locking effect, allergy relieving effect and skin whitening and color spot fading effect, is free of side effect and has low dependency.
Owner:董嘉丽
Vinpocetine transdermal patch and preparation method thereof
ActiveCN101822652AImprove solubilitySolve the problem of reduced skin penetration rateOrganic active ingredientsPharmaceutical non-active ingredientsTransdermal patchVinpocetine
The invention relates to a vinpocetine-contained transdermal patch and a preparation method thereof. The vinpocetine transdermal patch comprises five layers of a protecting layer, an adhesive layer, a release controlling film layer, a medicament reservoir and a back lining layer; the medicament reservoir contains 1-10 percent of vinpocetine or pharmaceutically acceptable salt of vinpocetine, 0-40percent of transdermal accelerating agent, a pH adjusting agent and the balance of reservoir substrate; the adhesive layer contains 0-8 percent of vinpocetine or pharmaceutically acceptable salt of vinpocetine, 0-40 percent of transdermal accelerating agent, a pH adjusting agent and the balance of pressure-sensitive adhesive; and pH values of the medicament reservoir and the adhesive layer are between 3.5 and 6.5. The invention solves the problems of less drug-loading amount, difficult formation of higher medicament concentration gradient and lower transmission rate of the traditional transdermal preparation by adopting a new preparation technology, has good transdermal effect and continuously releases medicaments for 1-7 days; and animal in-vivo tests show that the vinpocetine-contained transdermal patch has no simulating performance and sensitizing performance on skin.
Owner:HANGZHOU MINSHENG PHARM CO LTD
Triple high immunity immunoglobulin for anti swine pest, aujeszkys disease of pig, and parvovirus of pig
InactiveCN1958069ASignificant clinical effectStrong specificityAntiviralsAntibody ingredientsAdjuvantGlycoside formation
An immunoglobulin for preventing the cholera, pseudorabies and parvovirus of hog is prepared from their immunogens through proportional mixing them together and proportionally mixing it with complete Frennd's adjuvant. It features that the glycoside-peptide injection is used as its immunopotentiator.
Owner:张中洋
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com