Novel oriented synthesis method of voriconazole, medicinal salt and intermediate thereof
A voriconazole and directional synthesis technology, which is applied in the direction of pharmaceutical formulations, non-active ingredients of polymer compounds, medical preparations containing active ingredients, etc., can solve problems such as high cost, unfavorable environmental protection, and unsatisfactory directional synthesis selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
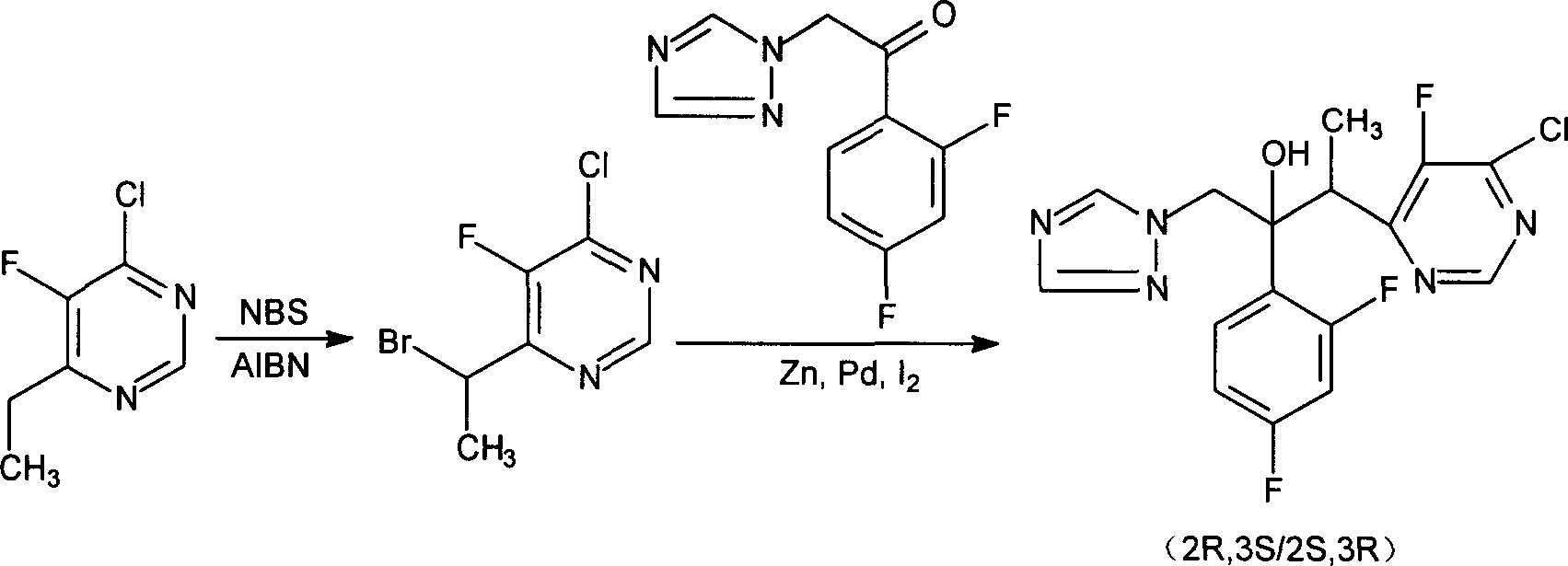
[0029] Embodiment 1: the preparation of double 4-chloro-5-fluoro-6-ethyl pyrimidine zinc
[0030] Install a mechanical stirrer, a thermometer, a reflux condenser and a gas introduction device in a 5L dry three-necked flask. Add 150.g (2.2mol) of copper-containing 5% zinc-copper alloy powder and 2000ml of anhydrous tetrahydrofuran to it successively, feed nitrogen, add 147g of 4-chloro-5-fluoro-6-(1-bromoethyl)pyrimidine (0.5mol), stirring and heating to reflux, after the reaction is initiated, add dropwise a 500ml tetrahydrofuran solution containing 440g (1.5mol) of 4-chloro-5-fluoro-6-(1-bromoethyl)pyrimidine to control the reaction rate Let it flow back smoothly. After the reaction was complete, cool, filter, and concentrate the filtrate to dryness. The residue was crystallized in a petroleum ether / diethyl ether mixed solvent, filtered, and dried to obtain 398 g of the target product bis-4-chloro-5-fluoro-6-ethylpyrimidine zinc. Yield: 81%, it can be directly used in the f...
Embodiment 2
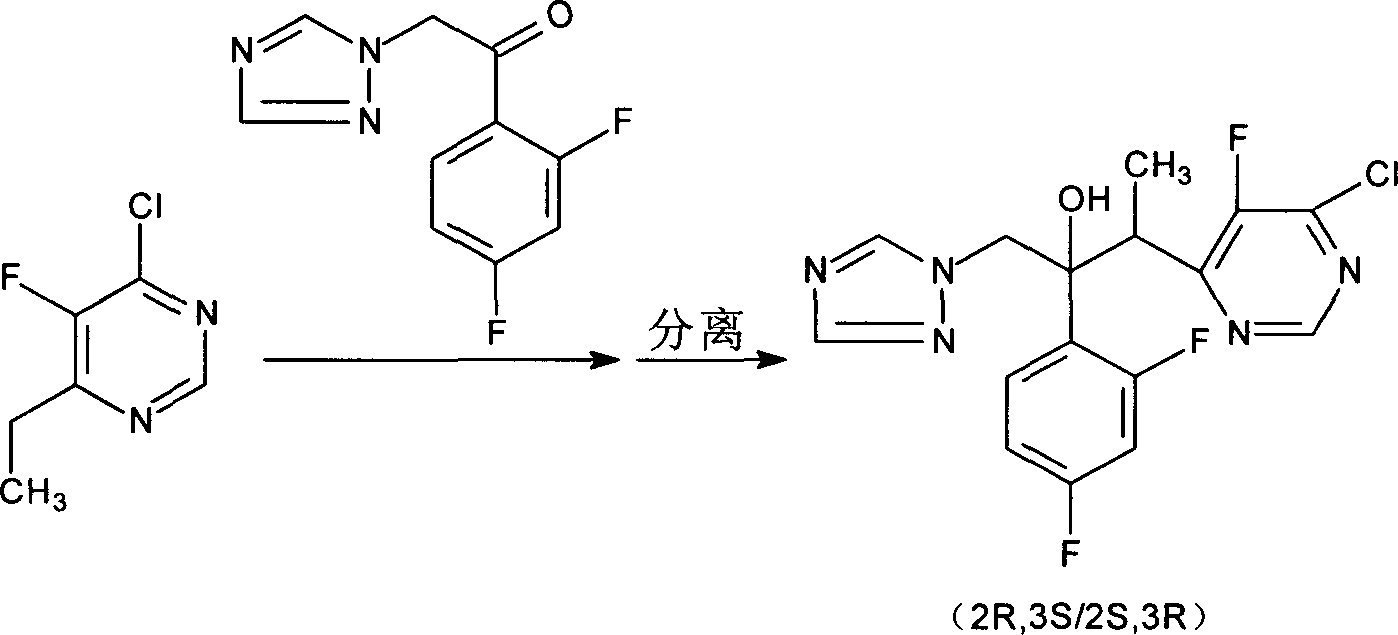
[0031] Example 2: (2R,3S / 2S,3R)-2-(2,4 difluorophenyl)-3-(4-chloro-5-fluoropyrimidin-6-yl)-1-(1H-1, Preparation of 2,4-triazol-1-yl)-2-butanol hydrochloride (Method A)
[0032]Install mechanical stirring, reflux condenser, dropping funnel and thermometer in a 5 L dry reaction flask. Add (R)-(+)-1,1'-bis-2-naphthol 143g (0.5mol), tetraisopropyl titanate 852g (3.0mol), bis-4-chloro-5-fluoro- 738g (1.5mol) of 6-ethylpyrimidine zinc and 2000ml of tetrahydrofuran were stirred for 1 hour, cooled to below 0°C, and 2H-(1,2,4-triazole)-2,4-difluoro Acetophenone (side chain) 558g (2.5mol) tetrahydrofuran solution 1000ml, make the reaction temperature not exceed 5 ℃. After the addition was complete, the reaction was continued at 0-5°C for 10 hours. After the reaction was completed, it was poured into an aqueous solution of acetic acid, and after stirring at room temperature for 10 minutes, the supernatant was poured out, and the solvent was evaporated under reduced pressure to recover...
Embodiment 3
[0033] Example 3: (2R,3S / 2S,3R)-2-(2,4 difluorophenyl)-3-(4-chloro-5-fluoropyrimidin-6-yl)-1-(1H-1, Preparation of 2,4-triazol-1-yl)-2-butanol hydrochloride (Method B)
[0034] Install mechanical stirring, reflux condenser, dropping funnel and thermometer in a 100ml dry reaction flask. Add (R)-(+)-5,5',6,6',7,7',8,8'-octahydro-1,1'-bis-2-naphthol 1.5g (0.005 mol), tetraisopropyl titanate 8.5g (0.03mol), bis-4-chloro-5-fluoro-6-ethylpyrimidine zinc 7.4g (0.015mol) and tetrahydrofuran 20ml, after stirring for 1 hour, cool to 0 Below ℃, add dropwise 10ml of tetrahydrofuran solution containing 5.6g (0.025mol) of 2H-(1,2,4-triazole)-2,4-difluoroacetophenone (side chain), so that the reaction temperature does not exceed 5 ℃. After the addition was completed, the reaction was continued at 0-5°C for 8 hours. After the reaction was completed, it was poured into an aqueous solution of acetic acid, and after stirring at room temperature for 10 minutes, the supernatant was poured out,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com