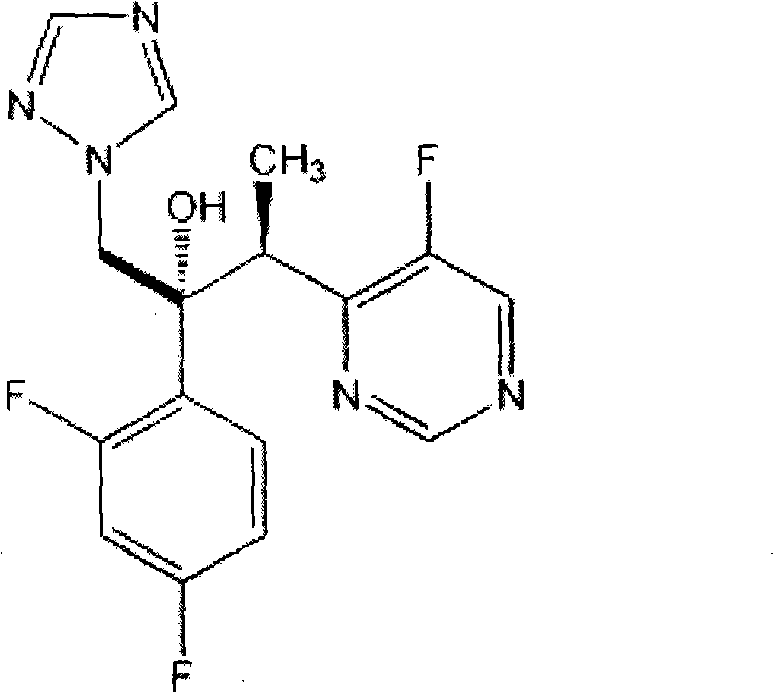
Voriconazole containing tablets
A voriconazole tablet and voriconazole technology, applied in the field of medicine, can solve the problems of tablet dissolution rate difference, low bioavailability, and hindering the treatment of patients, and achieve the effects of small dissolution fluctuation, small dissolution difference, and improved bioavailability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
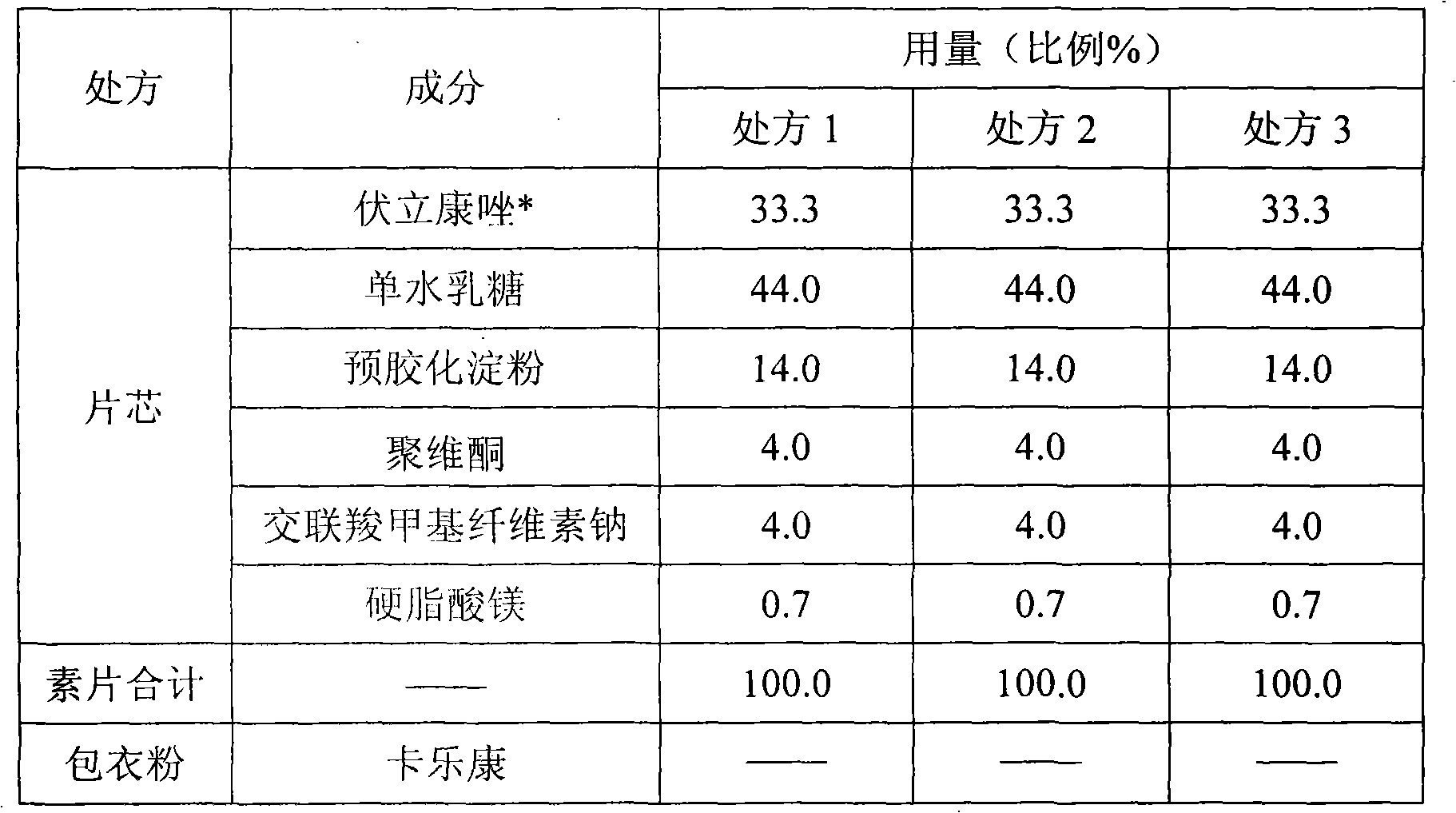
[0027] Embodiment 1 Voriconazole different particle size comparative experiments:
[0028]
[0029] * Voriconazole uses three particle sizes marked 1, 2, and 3 to show the difference. The particle size of voriconazole 1 used in prescription 1 is D (V,0.9) About 200 μm, the voriconazole particle size 2 used in prescription 2 is D (V,0.9) About 100 μm, the voriconazole particle size 3 used in prescription 3 is D (V,0.9) about 40 μm.
[0030] Preparation Process:
[0031] 1. Dissolve the prescribed amount of povidone in an appropriate amount of purified water to make an adhesive for later use;
[0032] 2. Put voriconazole, lactose monohydrate, pregelatinized starch, and croscarmellose sodium into a granulation pot and mix to obtain Mix A;
[0033] 3. Add the binder in step 1 into the granulation pot and granulate with the mixture A to obtain wet granules, granulate the wet granules and dry to obtain dry granules B;
[0034] 4. Add the prescribed amount of magnesium steara...
Embodiment 2
[0043] Embodiment 2 Comparative test of different disintegrant contents of voriconazole tablets
[0044]
[0045]
[0046]* Lactose monohydrate is the filler, and minor changes have little effect on the tablet. Therefore, the components of the excipients in prescriptions 4-6 are the same, and the changed amount of the disintegrant is supplemented by the filler lactose monohydrate accordingly. The preparation process is the same as in Example 1 Same, same control tablet disintegration time.
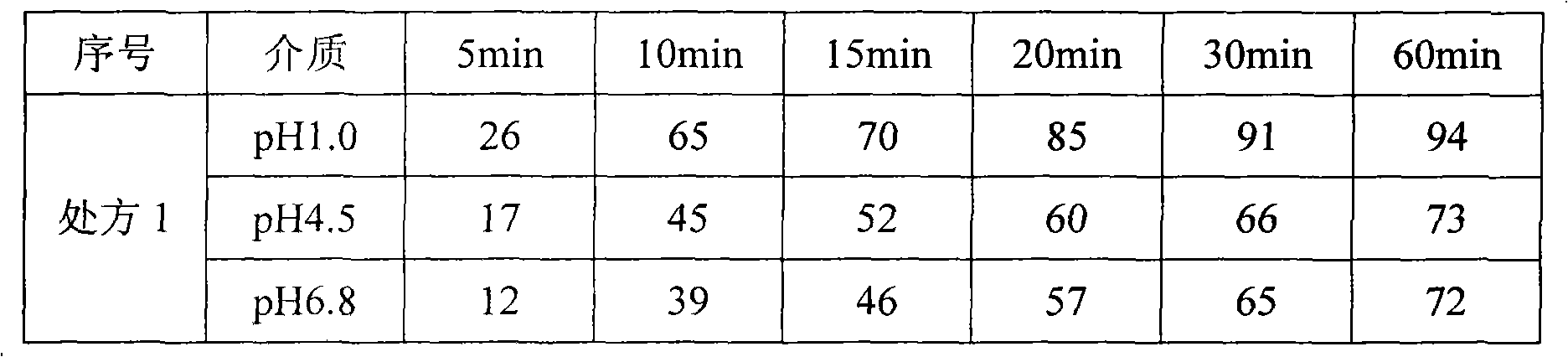
[0047] Table 3 Dissolution results of prescription 4-6 under pH1.0 dissolution medium
[0048] serial number
[0049] Result analysis: as seen from Table 3, along with the increase of disintegrant content in tablet, the stripping of tablet becomes faster. However, too few disintegrants prevent rapid dissolution, too many disintegrants, the tablet is more likely to absorb moisture, the dissolution is unstable after storage, and the quality of the drug cannot be guaranteed. ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com