New method for prepraring Voriconazole intermediate of antifungal drug
A technology for triazole derivatives and acid addition salts, which is applied in the field of preparation of triazole derivatives or their acid addition salts, and can solve the problems of inability to effectively control the optical purity of the final product and poor stereoselectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
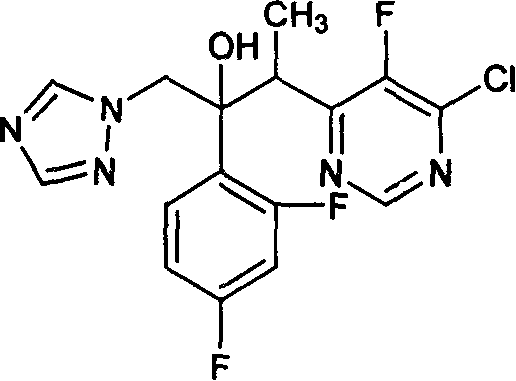
[0019] Preparation of (2R,3S / 2S,3R)-3-(4-chloro-5-fluoropyrimidin-6-yl)-2-(2,4-difluorophenyl)-1-(1H-1,2, 4-triazol-1-yl)butan-2-ol hydrochloride
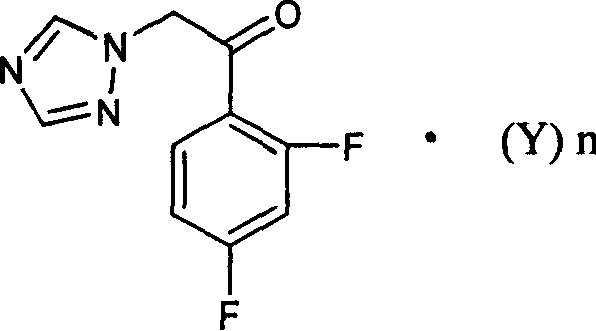
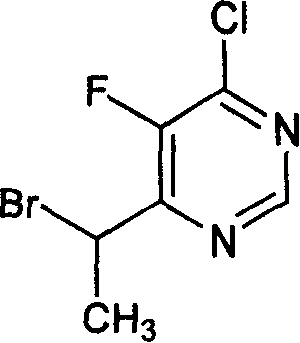
[0020] Under nitrogen protection, 32 ml of tetrahydrofuran, 11 g of zinc powder and 0.24 g of lead powder were put into a 200 ml reaction bottle, stirred, and heated to reflux for 3 hours. Cool to room temperature and stir for 16 hours. A solution of 4.4 g of iodine in 13 ml of tetrahydrofuran was added dropwise. After the dropwise addition was completed, the temperature was lowered, and 7.9 grams of 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazol-1-yl)ethanone camphorsulfonate and A solution of 4.2 g of 6-(1-bromoethane)-4-chloro-5-fluoropyrimidine in 32 ml of tetrahydrofuran. After the dropwise addition, the low-temperature reaction was continued for 2 hours, the temperature of the reaction system was warmed to room temperature, 5.2 g of glacial acetic acid and 50 ml of water were added, and stirred. Filter to remove metal residue...
Embodiment 2
[0022] Preparation of (2R,3S / 2S,3R)-3-(4-chloro-5-fluoropyrimidin-6-yl)-2-(2,4-difluorophenyl)-1-(1H-1,2, 4-triazol-1-yl)butan-2-ol hydrochloride
[0023] Under nitrogen protection, 64 ml of tetrahydrofuran, 22 g of zinc powder and 0.48 g of lead powder were put into a 500 ml reaction bottle, stirred, and heated to reflux for 3 hours. Cool to room temperature and stir overnight. A solution of 9 g of iodine in 25 ml of tetrahydrofuran was added dropwise. After the dropwise addition, the temperature was lowered, and 9.2 grams of 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazol-1-yl)ethanone hydrochloride and 8.5 A solution of 6-(1-bromoethane)-4-chloro-5-fluoropyrimidine in 65 ml THF. After the dropwise addition, continue the reaction at low temperature for 2 hours, warm the temperature of the reaction system to room temperature, add 10 g of glacial acetic acid and 100 ml of water, and stir. Filter to remove metal residues. The organic solvent was removed by rotary evaporation. ...
Embodiment 3
[0025] Preparation of (2R,3S / 2S,3R)-3-(4-chloro-5-fluoropyrimidin-6-yl)-2-(2,4-difluorophenyl)-1-(1H-1,2, 4-triazol-1-yl)butan-2-ol hydrochloride
[0026] Under nitrogen protection, 48 ml of tetrahydrofuran, 16.5 g of zinc powder and 0.36 g of lead powder were put into a 500 ml reaction bottle, stirred, and heated to reflux for 3 hours. Cool to room temperature and stir overnight. A solution of 6.6 g of iodine in 20 ml of tetrahydrofuran was added dropwise. After the dropwise addition, the temperature was lowered, and 6.8 grams of 1-(2,4-difluorophenyl)-2-(1H-1,2,4-triazol-1-yl)ethanone hydrochloride and 6.3 A solution of 6-(1-bromoethane)-4-chloro-5-fluoropyrimidine in 48 ml THF. After the dropwise addition, the low-temperature reaction was continued for 2 hours, and the temperature of the reaction system was warmed to room temperature, 8 g of glacial acetic acid and 75 ml of water were added, and stirred. Filter to remove metal residues. The organic solvent was removed ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com