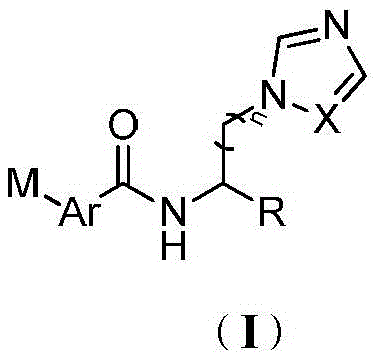
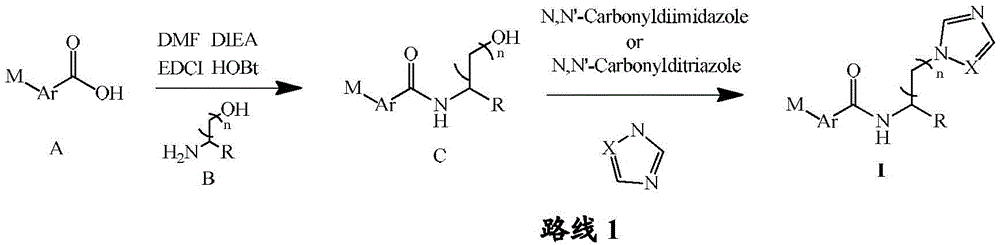
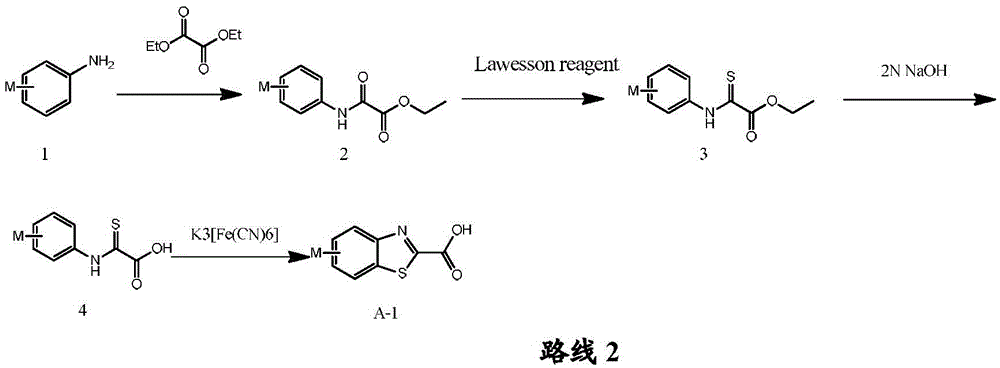
Acylaminoimidazole derivative and use thereof
A carboxamide and thiazole technology, applied in the field of amidoimidazole derivatives and their uses, can solve the problems of single dosage form, narrow antibacterial spectrum, drug interaction, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0330] Example 1: Preparation of (S)-2-(benzo[d]thiazole-2-carboxamido)-3-(1H-imidazol-1-yl)propionic acid methyl ester
[0331] Preparation of step A-12-oxo-2-phenylaminoacetic acid ethyl ester (1)
[0332] 5.00 g (53.8 mmol) of aniline was added to 20 mL of diethyl oxalate, and the temperature was controlled at 150° C. After 5 hours, TLC monitored that the reaction was complete. The reaction solution was cooled, 100 mL of petroleum ether was added, and a large amount of white solid was formed after stirring for 10 min, and 8.52 g of white solid was obtained by suction filtration, with a yield of 82.1%. MS[M+H]+ + (m / z): 194.
[0333] Preparation of step A-22-thio-2-phenylaminoacetic acid ethyl ester (2)
[0334] Add 2.00 g (10.4 mmol) of intermediate 1 (ethyl 2-oxo-2-phenylaminoacetate) and 2.10 g (5.2 mmol) of Lawson’s reagent into 30 mL of toluene, react at 70° C. for 3 h, and monitor the completion of the reaction by TLC. The solvent was concentrated under reduced pre...
Embodiment 2
[0346] Example 2 (S)-2-(benzo[d]thiazole-2-carboxamido)-3-(1H-imidazol-1-yl) ethyl propionate
[0347] ESI-MS[M+H] + (m / z):345;
Embodiment 3
[0348] Example 3 (S)-2-(benzo[d]thiazole-2-carboxamido)-3-(1H-imidazol-1-yl)propionic acid propyl ester
[0349] ESI-MS[M+H] +(m / z):359;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com