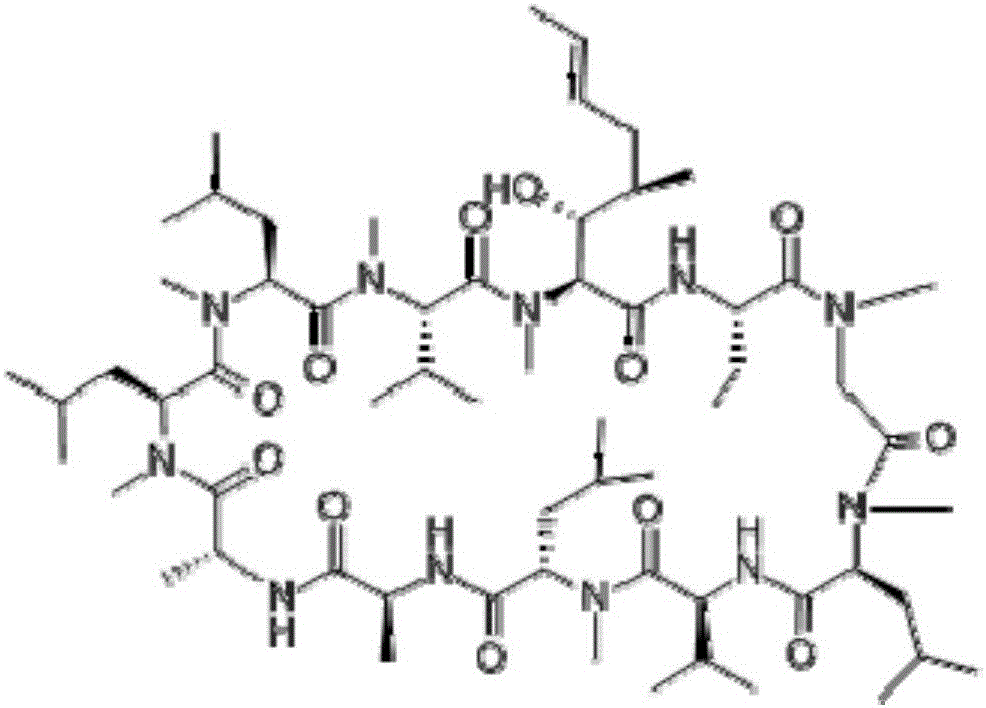
Ophthalmic ciclosporin emulsion
A cyclosporine and emulsion technology, which is applied in the directions of cyclic peptide components, emulsion delivery, medical preparations with inactive ingredients, etc. problem, to achieve the effect of improving targeting, increasing the content of active ingredients, and improving targeting
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0014] The cyclosporine ophthalmic emulsion in the embodiment of the present invention and comparative example is prepared according to the following method
[0015] 1) Mix polyoxyethylene hydrogenated castor oil and medium-chain fatty acid glycerides, heat to 50-70°C, add cyclosporine and dissolve to obtain an oil phase;
[0016] 2) Dissolving other components in water to obtain a water phase, heating the water phase to 50-70° C., adding the oil phase while stirring the water phase with a homomixer to obtain colostrum. Finally, the colostrum is passed through a high-pressure milk homogenizer, and then sterilized by filtration to obtain cyclosporine ophthalmic emulsion.
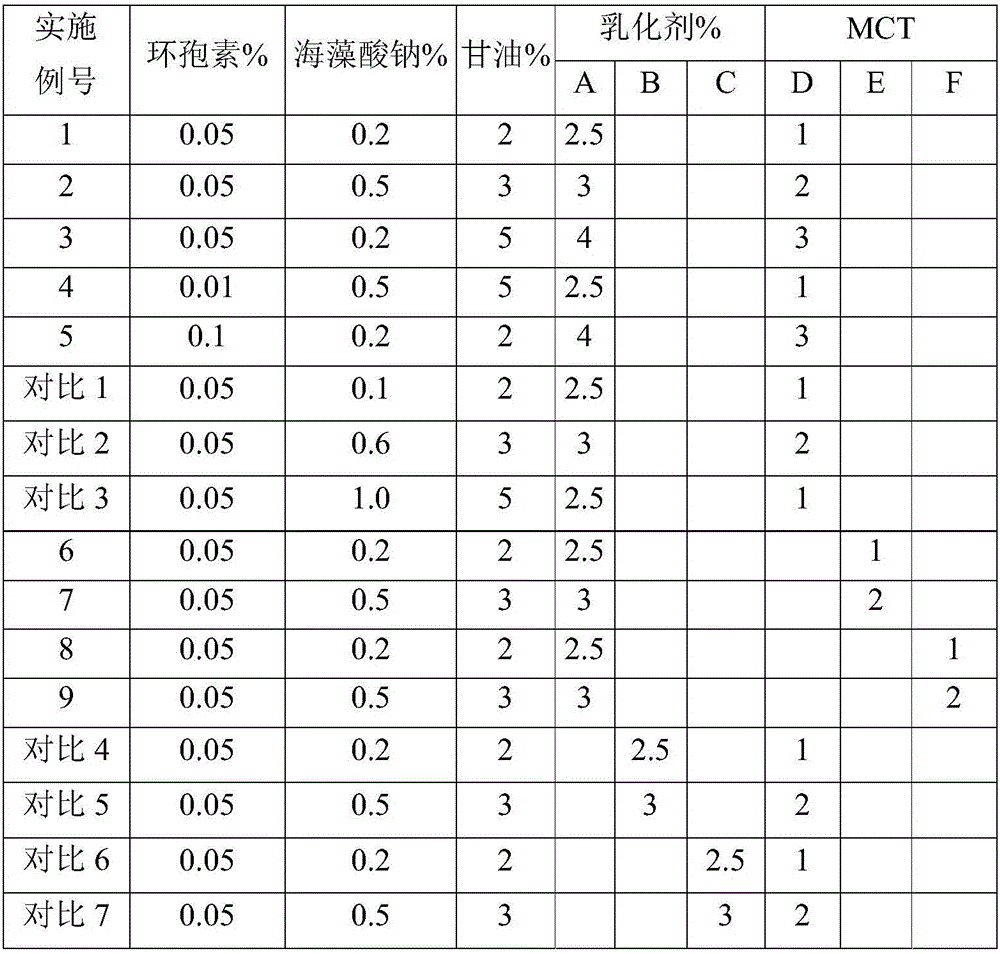
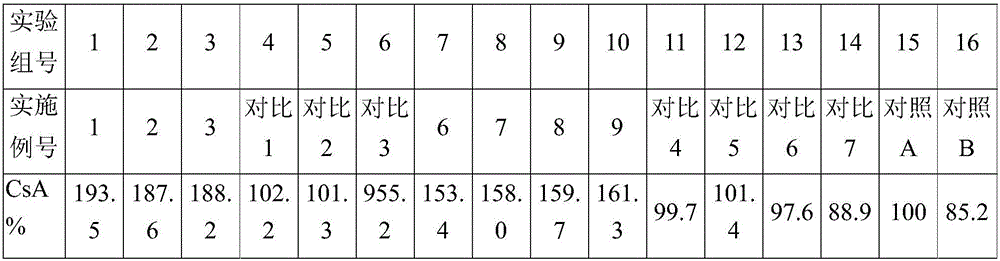
[0017] The formulas of all embodiments and comparative examples are shown in the following table, wherein emulsifier A is polyoxyethylene hydrogenated castor oil, emulsifier B is polyoxyethylene castor oil, and emulsifier C is Tween-80; MCTD is caprylic triglyceride, MCTE is capric triglyceride, MCTF is capr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com