Preparation method of ciclosporin ophthalmic solution
An ophthalmic solution, cyclosporine technology, applied in the direction of cyclopeptide components, pharmaceutical formulations, sensory diseases, etc., to achieve the effect of improving compliance and tolerance, reducing irritation, and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
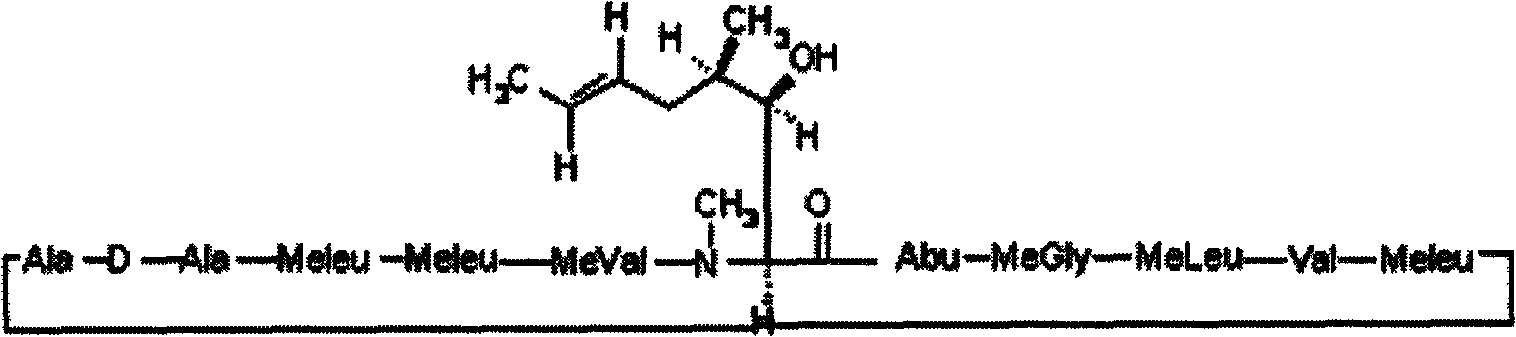
[0033] Embodiment 1 cyclosporine ophthalmic solution 1 and its preparation
[0034] The prescription involved in this patent is as follows:
[0035] 1) Prescription:
[0036]
[0037] 2) Preparation method:
[0038] (1) Cyclosporin is added to medium-chain triglycerides to dissolve it;
[0039] (2) add benzalkonium chloride in aqueous phase, make it dissolve;
[0040] (3) Add mannitol to the water phase to dissolve it;
[0041] (4) polyoxyethylene hydrogenated castor oil 40 Add n-butanol and glycerol to the water phase to dissolve them;
[0042] (5) Under stirring, add the oil phase to the water phase and mix well;
[0043] (6) Replenish with water to the full amount, ready to serve.
Embodiment 2
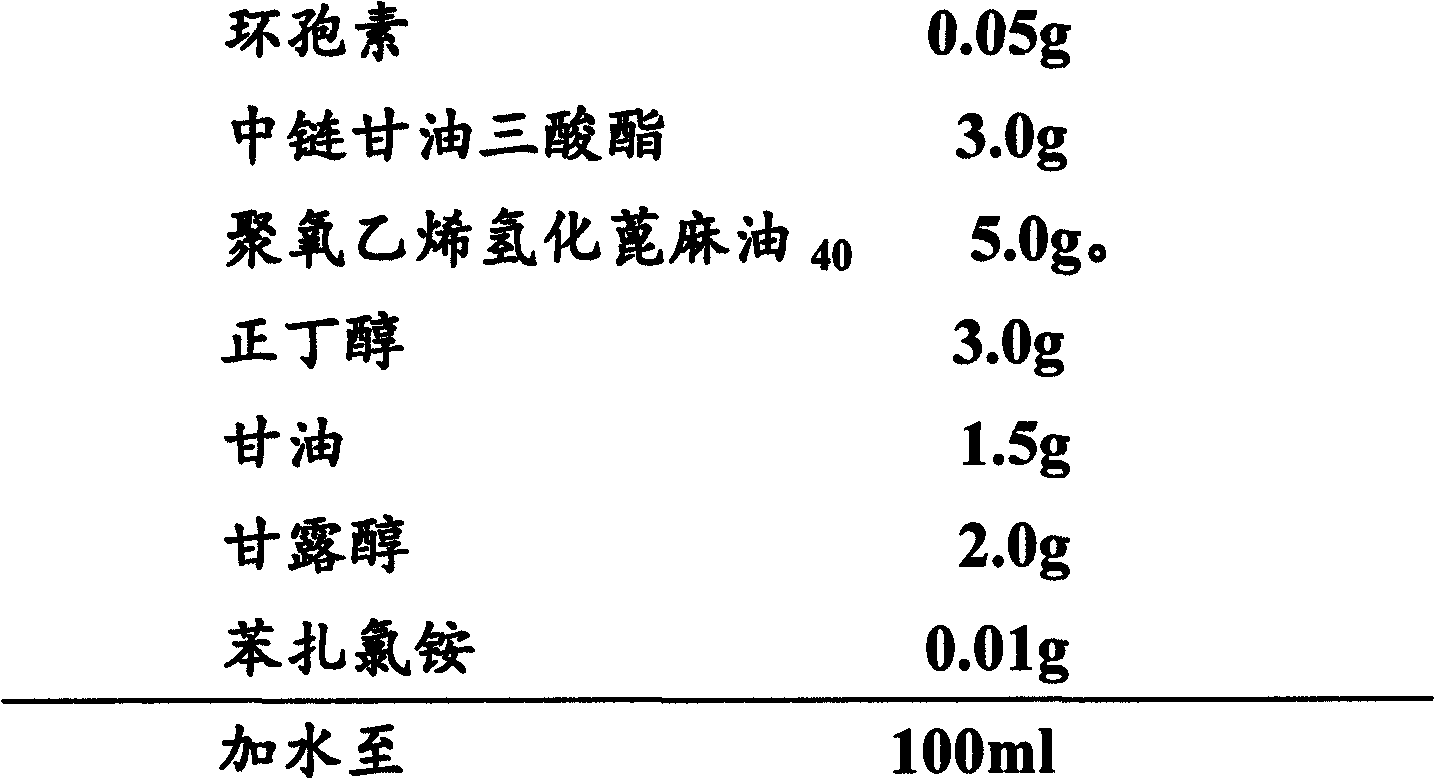
[0044] Embodiment 2 cyclosporine ophthalmic solution 2 and its preparation
[0045] 1) Prescription:
[0046]
[0047] 2) Preparation method:
[0048] (1) Cyclosporin is added to medium-chain triglycerides to dissolve it;
[0049] (2) add benzalkonium bromide in aqueous phase, make it dissolve;
[0050] (3) Add sorbitol to the water phase to dissolve it;
[0051] (4) Add Tween-80, propylene glycol and sodium hyaluronate to the water phase to dissolve them;
[0052] (5) Under stirring, add the oil phase to the water phase and mix well;
[0053] (6) Replenish with water to the full amount, ready to serve.
Embodiment 3
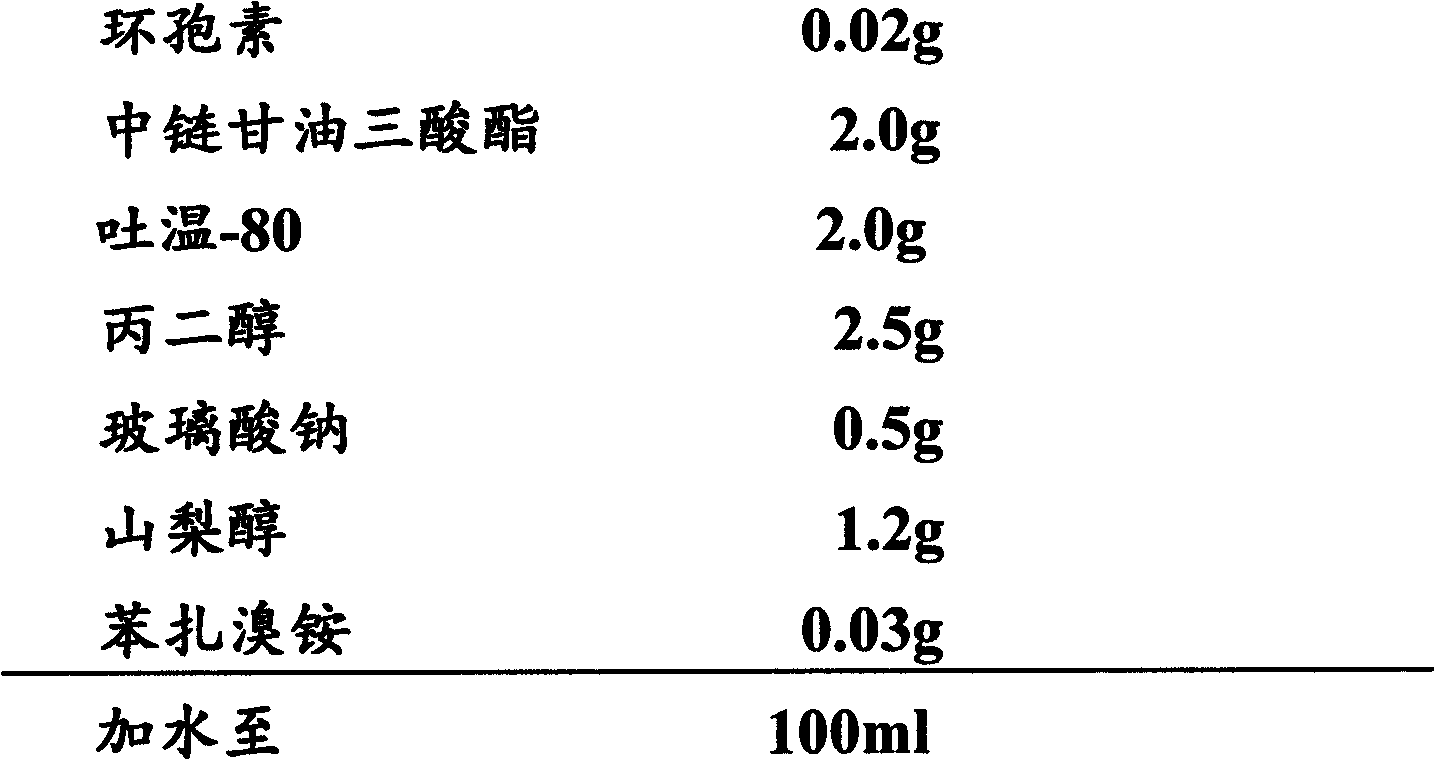
[0054] Embodiment 3 cyclosporine ophthalmic solution 3 and its preparation
[0055] 1) Prescription:
[0056]
[0057] 2) Preparation method:
[0058] (1) Cyclosporin is added to the phthalate to dissolve it;
[0059] (2) add cetrimonium bromide in aqueous phase, make it dissolve;
[0060] (3) Add mannitol to the water phase to dissolve it;
[0061] (4) polyoxyethylene hydrogenated castor oil 40 Add propylene glycol and glycerin to the water phase to dissolve it;
[0062] (5) Under stirring, add the oil phase to the water phase and mix well;
[0063] (6) Replenish with water to the full amount, ready to serve.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com