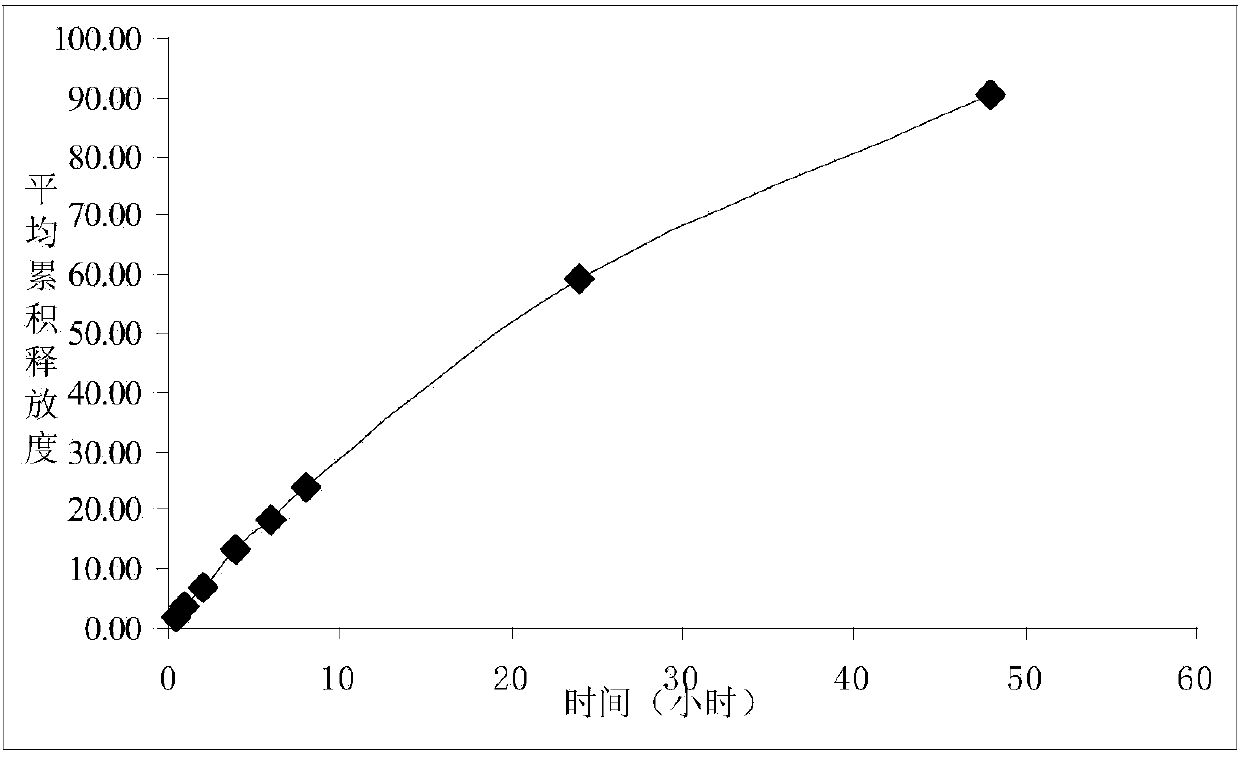
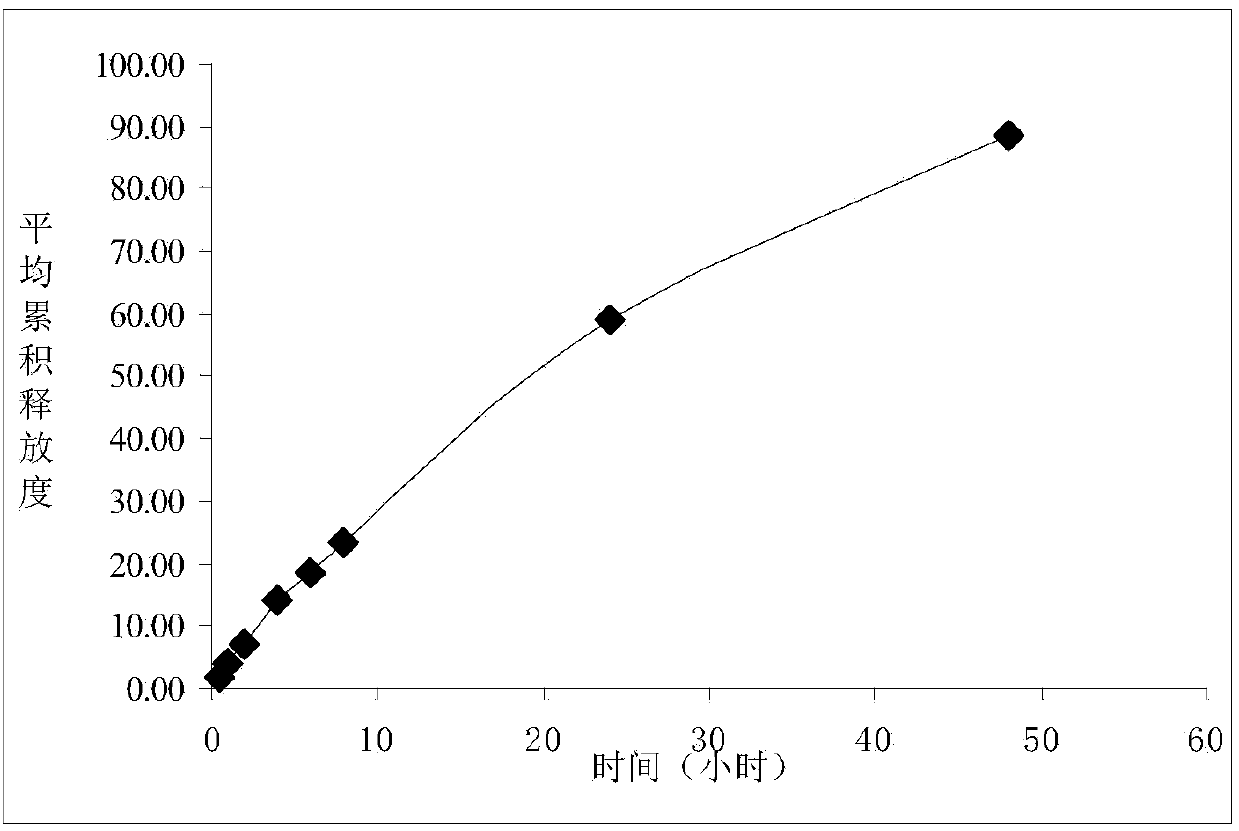
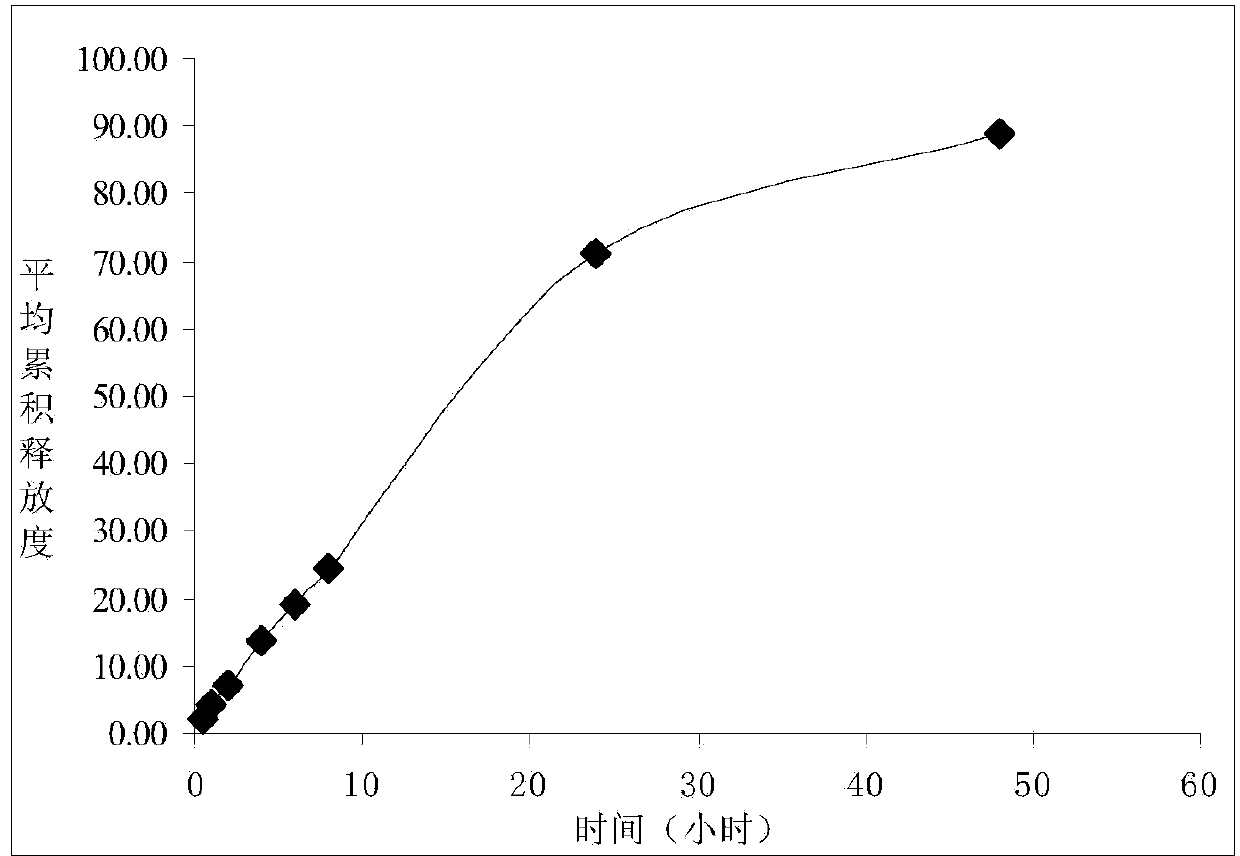
Ciclosporin eye gel and preparation method thereof
A technology of cyclosporine and gel, which is applied in the field of medicine, can solve the problems of short corneal retention time, low bioavailability, and intolerance of patients, and achieve long corneal retention time, high bioavailability, and low damage Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0048] The present invention also provides a method for preparing the cyclosporin eye gel, which specifically comprises mixing the aqueous gel matrix with water to prepare the gel matrix, and then mixing the cyclosporin A with a nonionic surfactant and a wetting agent It is uniformly mixed and stirred evenly with the prepared gel matrix, pH regulator is added to adjust the pH to 5.0-8.0, sterilized, and then sterilized into eye drop bottles to obtain cyclosporine eye gel.
[0049] Sterilization refers to the use of physical or chemical methods to kill all microorganisms, including pathogenic and non-pathogenic microorganisms and spores, so that they can reach the level of sterility protection. The sterilization of the present invention is preferably autoclaving.
[0050] Autoclave sterilization is a method of heating with saturated steam with a pressure greater than normal pressure in a closed sterilization container for sterilization. The autoclave sterilization method of the pre...
Embodiment 1
[0054] Example 1: The cyclosporine eye gel of the present invention
[0055] prescription:
[0056] An eye gel containing 0.01% cyclosporine A, the specific prescription is as follows:
[0057] Cyclosporine A 0.01% by weight, Tween 802% by weight, Carbomer 0.01% by weight, Propylene glycol 5% by weight, appropriate amount of sodium hydroxide, add water for injection to 100g, and make a 100g specification containing 0.01% cyclosporin eye gel .
[0058] Preparation:
[0059] 1) Preparation of gel matrix
[0060] Take the prescribed amount of water for injection into a beaker, and spread the prescribed amount of carbomer on the surface of the above-mentioned water for injection, and let it swell at rest.
[0061] 2) Preparation of cyclosporin A solution
[0062] Accurately weigh cyclosporin A, add Tween 80 to dissolve cyclosporin A, add propylene glycol, and stir well.
[0063] 3) Mixing and post-processing
[0064] Take the gel matrix prepared in step (1) and the drug solution prepared in ste...
Embodiment 2
[0069] prescription:
[0070] An eye gel containing 0.04% cyclosporine A, the specific prescription is as follows:
[0071] Cyclosporin A 0.04% by weight, castor oil 10% by weight, carbomer 0.2% by weight, propylene glycol 30% by weight, appropriate amount of sodium hydroxide, add water for injection to 100g, make a 100g specification containing 0.04% cyclosporin A eye gel glue.
[0072] Preparation:
[0073] 1) Preparation of gel matrix
[0074] Take the prescribed amount of water for injection into a beaker, and spread the prescribed amount of carbomer on the surface of the above-mentioned water for injection, and let it swell at rest.
[0075] 2) Preparation of cyclosporin A solution
[0076] Accurately weigh cyclosporin A, add castor oil to dissolve cyclosporin A, add propylene glycol, and stir well.
[0077] 3) Mixing and post-processing
[0078] Take the gel matrix prepared in step (1) and the drug solution prepared in step (2), mix and stir uniformly, add a pH regulator to adjust th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com