Ophthamological drugs
a technology of ophthamological drugs and ophthalmic drugs, which is applied in the field of ophthamological drugs, can solve the problems of loss of vision, elevated intraocular pressure, and elevated pressure, and achieve the effect of increasing their penetration through the cornea
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
[0089] Preparation of 5-O-Ribitol[2,3-dichloro-4-(thiophene-2-carbonyl)]phenoxyacetate (Formula III), with reference to Scheme I.
[0090] To a stirring suspension of ticrynafen (0.40 g, 1.2 mmol) in anhydrous benzene (1.5 mL) was added thionyl chloride (0.18 mL, 2 equiv) at room temperature. The reaction was heated to reflux, at which time all ticrynafen dissolved. Reflux was maintained for 2.5 h and the reaction was cooled and concentrated at reduced pressure. The residual oil was resuspended in anhydrous THF (2 mL), and added dropwise at room temperature to a stirring solution of 1,2:3,4-di-O-isoproylideneribitol (0.28 g), triethylamine (0.57 mL) in THF (1 mL). The reaction was stirred at room temperature for 16 h, concentrated at reduced pressure and resuspended in EtOAc (100 mL). This solution was extracted with water (2×50 mL) and brine (50 mL), and dried (anhydrous MgSO4). The solvents were evaporated in vacuo, and the crude products were purified by flash chromatography (elut...
example 2
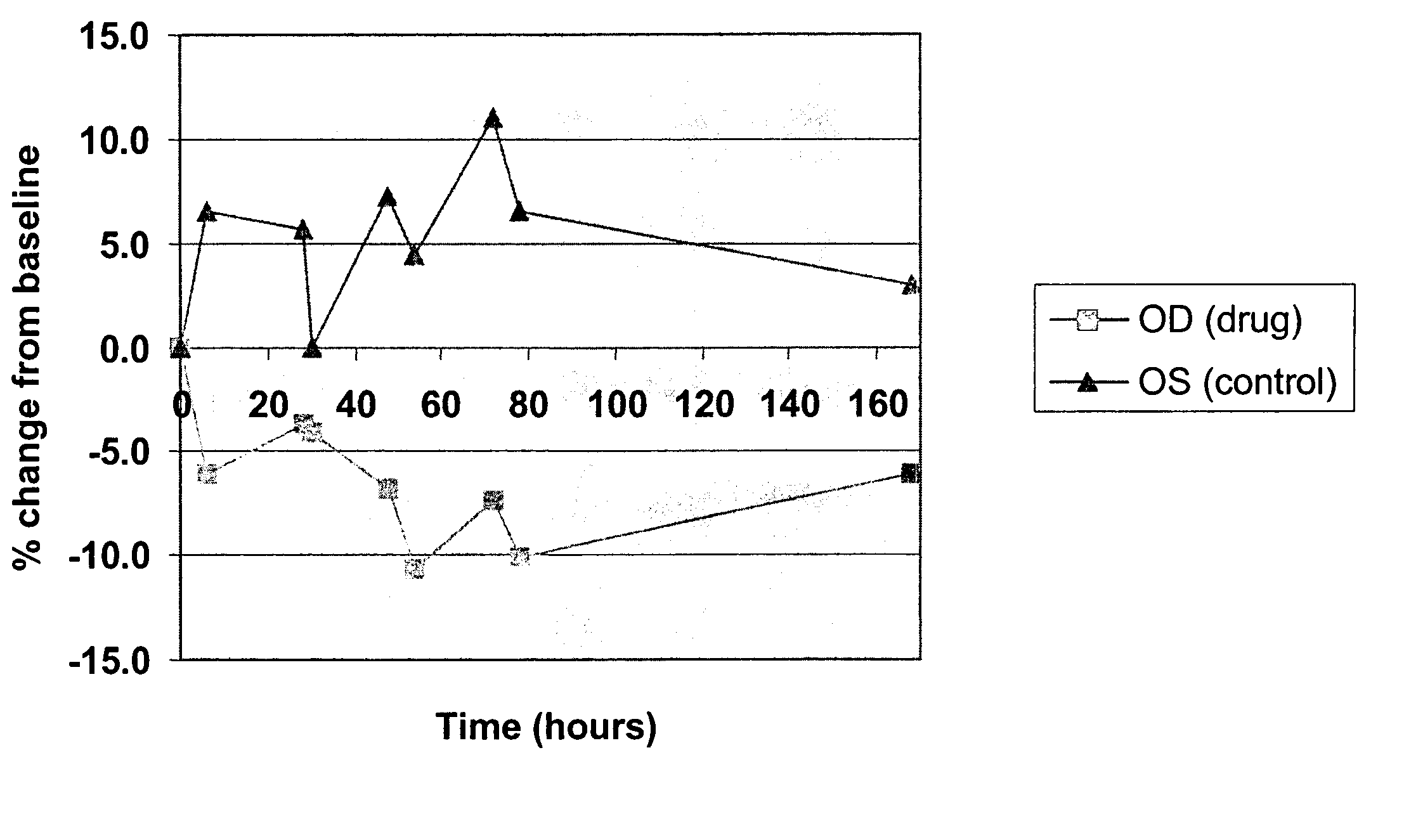
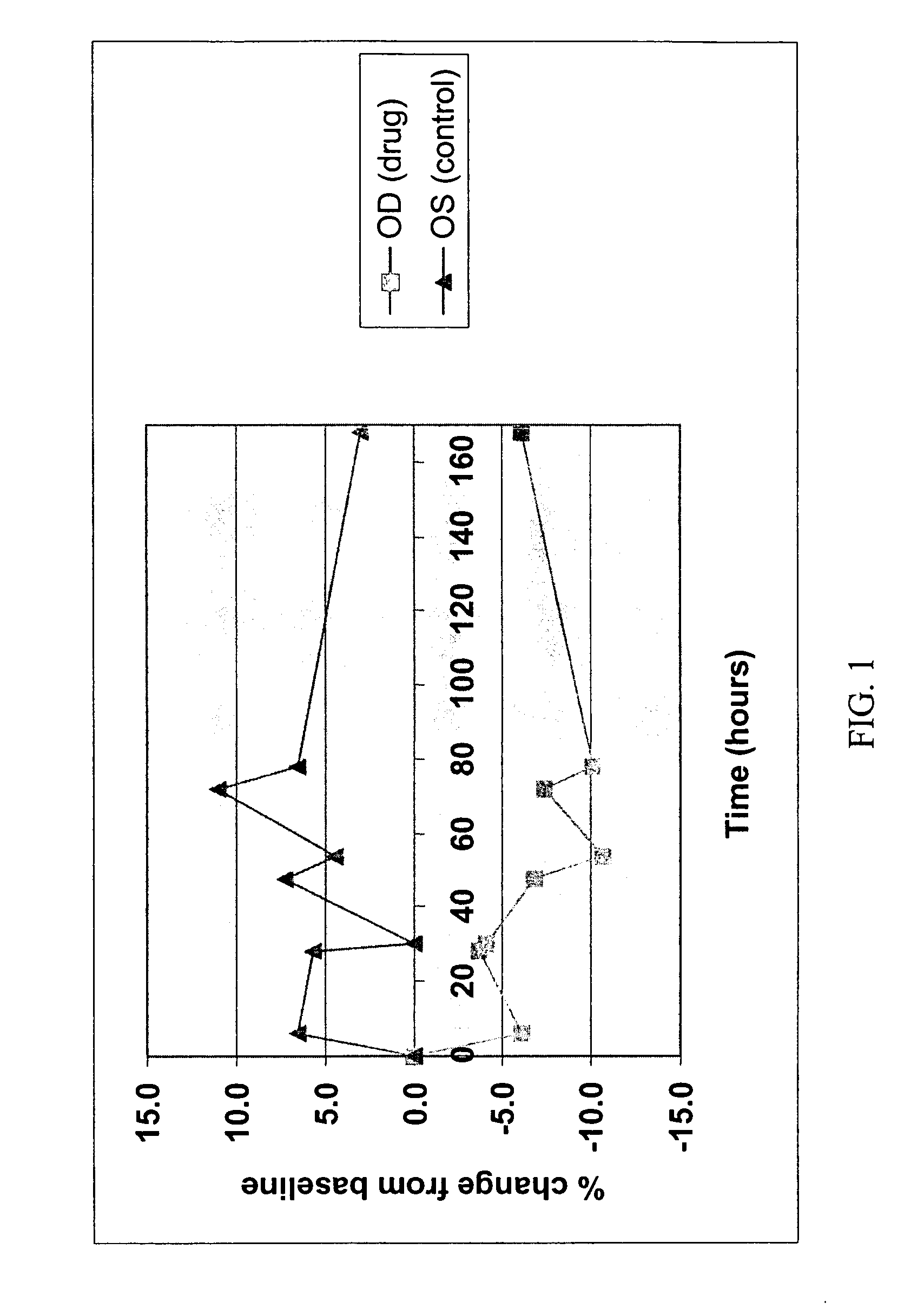
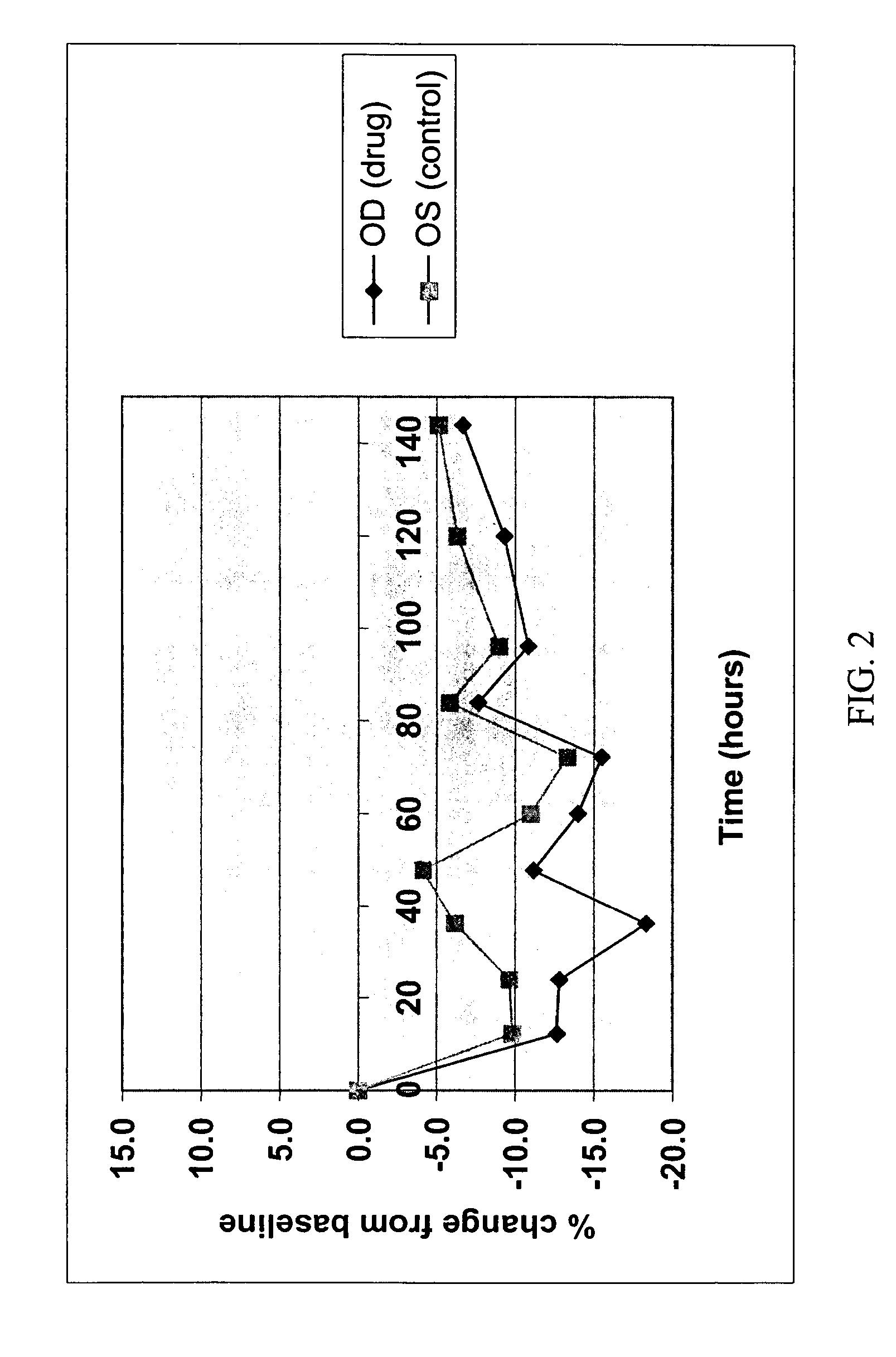
[0092] Ticrynafen (Formula IV) is a drug of known activity against glaucoma. The free acid, as the carboxylate salt, has little or no activity presumably because penetration through the cornea into the anterior chamber is negligible. Simple esters of ticrynafen are highly insoluble and also show little or no activity. In contrast, the ribitol ester of ticrynafen (Formula III) is completely soluble in petrolatum / lanolin mixtures. The compound reduces intraocular pressure (IOP) in a dose-dependent fashion in Dutch-Belted white rabbits (see Example 3 and FIGS. 1-4).
example 3
[0093] Ticrynafen-ribitol ester was formulated as an ointment in 5, 10, and 15% concentrations. The Dutch Belted rabbit model was used to test the formulation for both pressure lowering and side effects.
[0094] Baseline intraocular pressures were obtained by placing one drop of proparacaine in each eye followed by pressure measurements utilizing a Pneumotonometer®. One eye of each rabbit was then given 0.1 mg of ticrynafen-ribitol ester ointment topically in the inferior conjunctival sac. The second eye was used as a control with application of lanolin ointment without drug. Repeat doses were given at 24 hour intervals for a total of three doses (0, 24, and 48 hours). Intraocular pressures were recorded every 12 hours for one week. A total of twelve rabbits received the 5% ointment, eight rabbits the 10% ointment, and eight rabbits the 15% ointment.
[0095] Pressure reduction for each concentration is shown in FIGS. 1, 2 and 3. FIG. 4 presents the 10% data with statistically signific...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
| Time | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com