Derivatives of new chitosan, preparation method, and application in use for making ophthalmic preparation
A chitosan derivative, chitosan technology, applied in the fields of polymer chemistry and pharmaceutical preparations, can solve problems affecting yield and so on
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0100] Synthesis of temperature-sensitive chitosan derivative I:
[0101] The degree of deacetylation of chitosan is above 90%, and the viscosity average molecular weight is 60,000. The reagents used were analytically pure and chemically pure. The molecular weight cut-off of the dialysis bag is 10000 (MWCO=10 000).
[0102] 1.1 Synthesis of carboxylic acid derivatives (PNIPAAm-COOH) of poly(N-isopropylacrylamide)
[0103]
[0104] Add 10ml of methanol solution into the three-necked flask, raise the temperature to 70°C under nitrogen protection, slowly add N-isopropylacrylamide (IPN, 1g, 8.9mmol), azobisisobutyronitrile (AIBN, 20mg, 0.12mmol) and 3-mercaptopropionic acid (3-MPA, 0.2ml, 0.11mmol), continue to pass nitrogen, and stir at 70°C for 24h, evaporate the solvent, dissolve the mixture with 20ml of acetone, add 1ml of n-hexane dropwise, and filter the precipitate. Vacuum-dried at 40°C to obtain 980 mg of PNIPAAm-COOH.
[0105] The average molecular weight of the prod...
Embodiment 2
[0111] Determination of Phase Transition Temperature LCST of In Situ Gel Solution
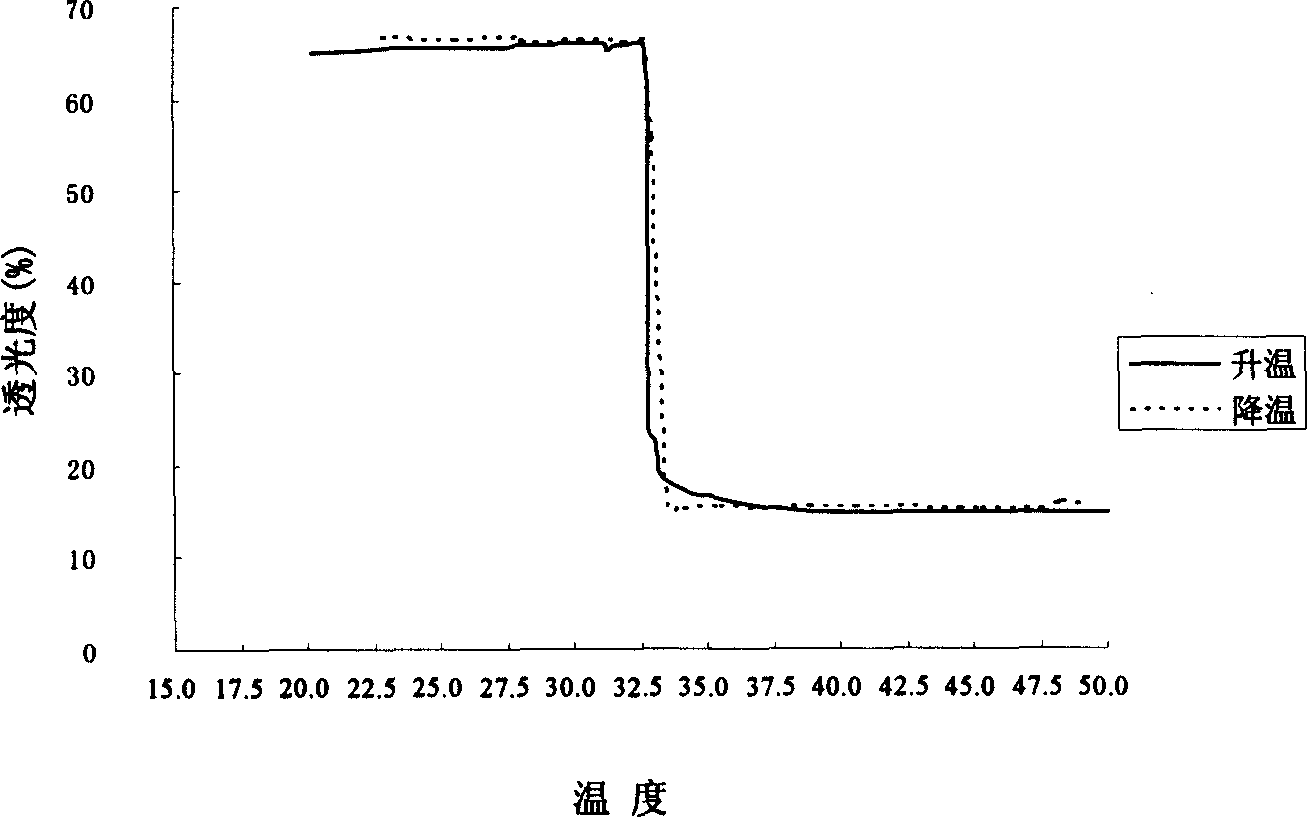
[0112] Prepare a 5 mg / ml copolymer solution, stir at room temperature for 24 hours, put it into a polystyrene cuvette, and measure the light transmittance on an ultraviolet spectrophotometer. Put the sample cell in a water bath and heat it to the measurement temperature, put it into the sample cell, and measure the light transmittance at 500nm. The temperature is measured every 1°C from 26-40°C.
[0113] Such as figure 2 , when the temperature is 32-33 ° C, the synthesized chitosan derivative undergoes a phase transition rapidly, and the phase transition is reversible, so it can be quickly transformed into a gel when applied to the eyes. The phase change that occurs when the storage temperature rises will restore its original properties as long as the temperature is properly cooled.
Embodiment 3
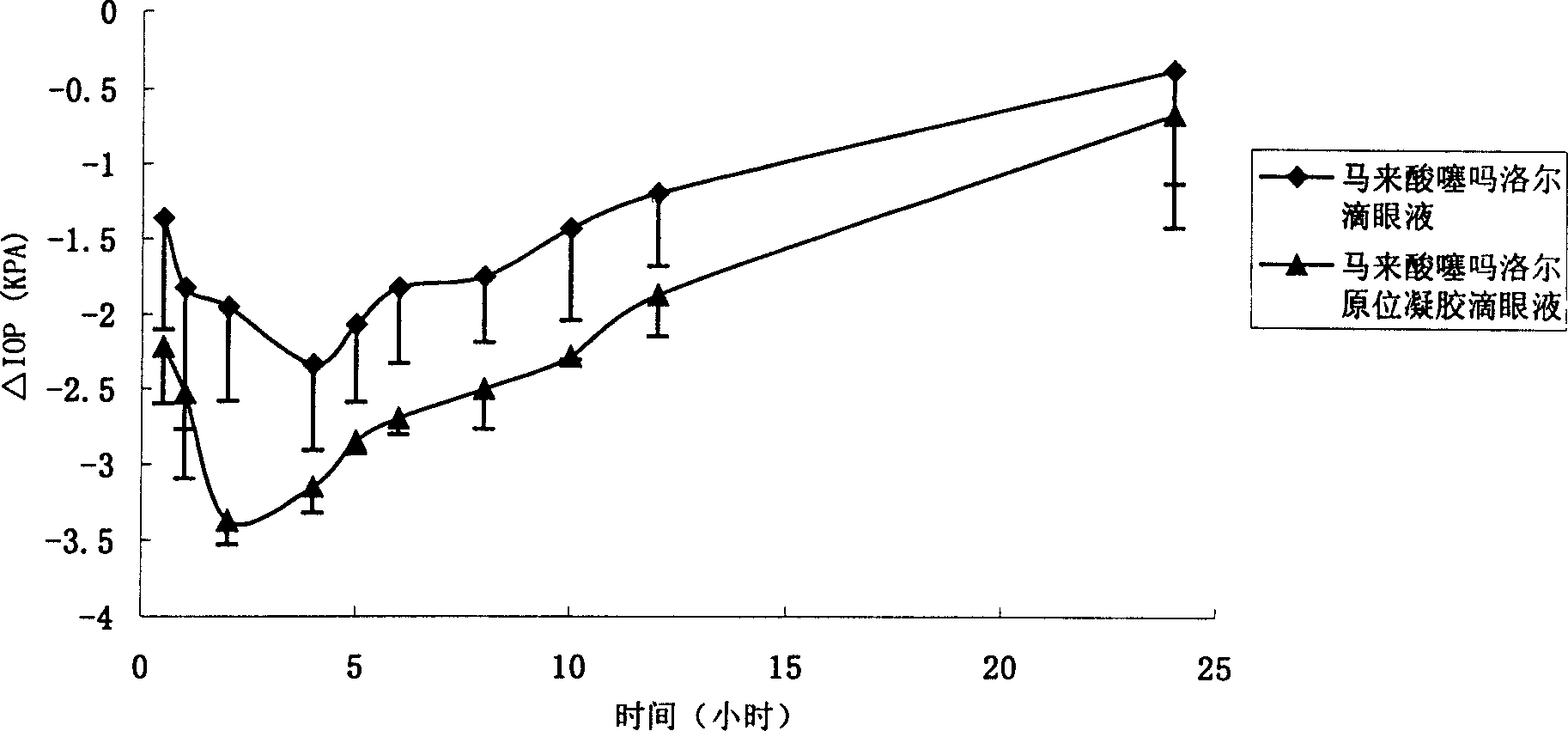
[0115] Timolol maleate in situ gel eye drops and preparation method thereof
[0116] Temperature-sensitive timolol maleate in situ gel eye drops 1000ml, its composition is 5.0g timolol maleate, temperature-sensitive chitosan derivative I 0.4g, sodium chloride 9g, benzalkonium Ammonium bromide 0.12g, sodium carbonate to adjust the pH, and the rest are sterilized water for injection.
[0117] Add appropriate amount of sterilized water for injection to a temperature-sensitive chitosan derivative, stir to make it fully swell, and make a blank gel solution; in addition, add timolol maleate, sodium chloride, and benzalkonium bromide Ammonium, dissolved in sterilized water for injection, stirred to dissolve, filtered, added the filtrate to the blank gel solution, mixed, and adjusted to pH 7 with sodium carbonate. Add sterilized water for injection to a sufficient amount, stir to form a uniform solution, and the above operations are all carried out under sterile conditions below 30°C...
PUM
| Property | Measurement | Unit |
|---|---|---|
| transition temperature | aaaaa | aaaaa |
| degree of substitution | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com