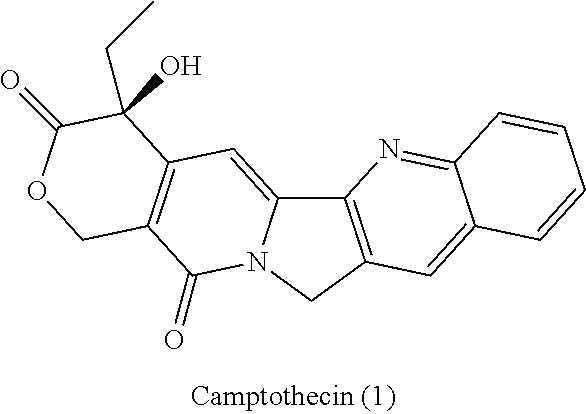
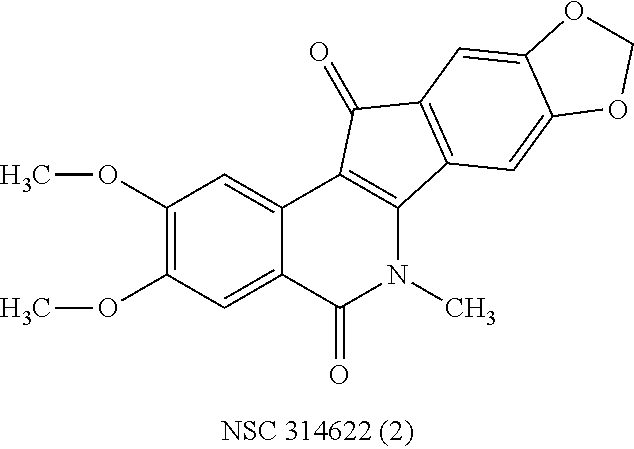
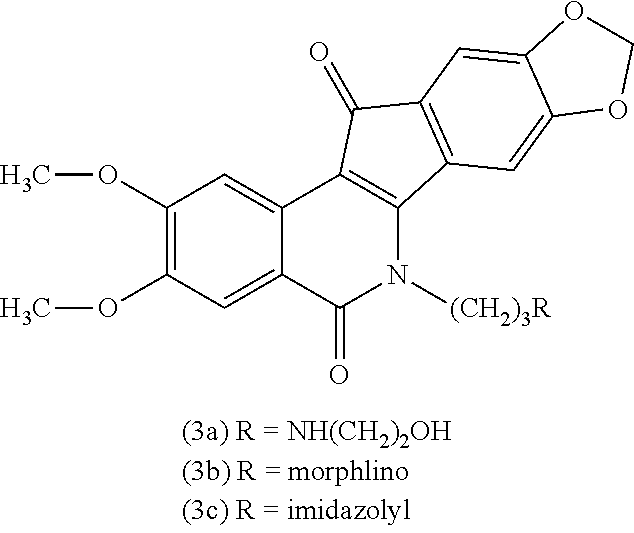
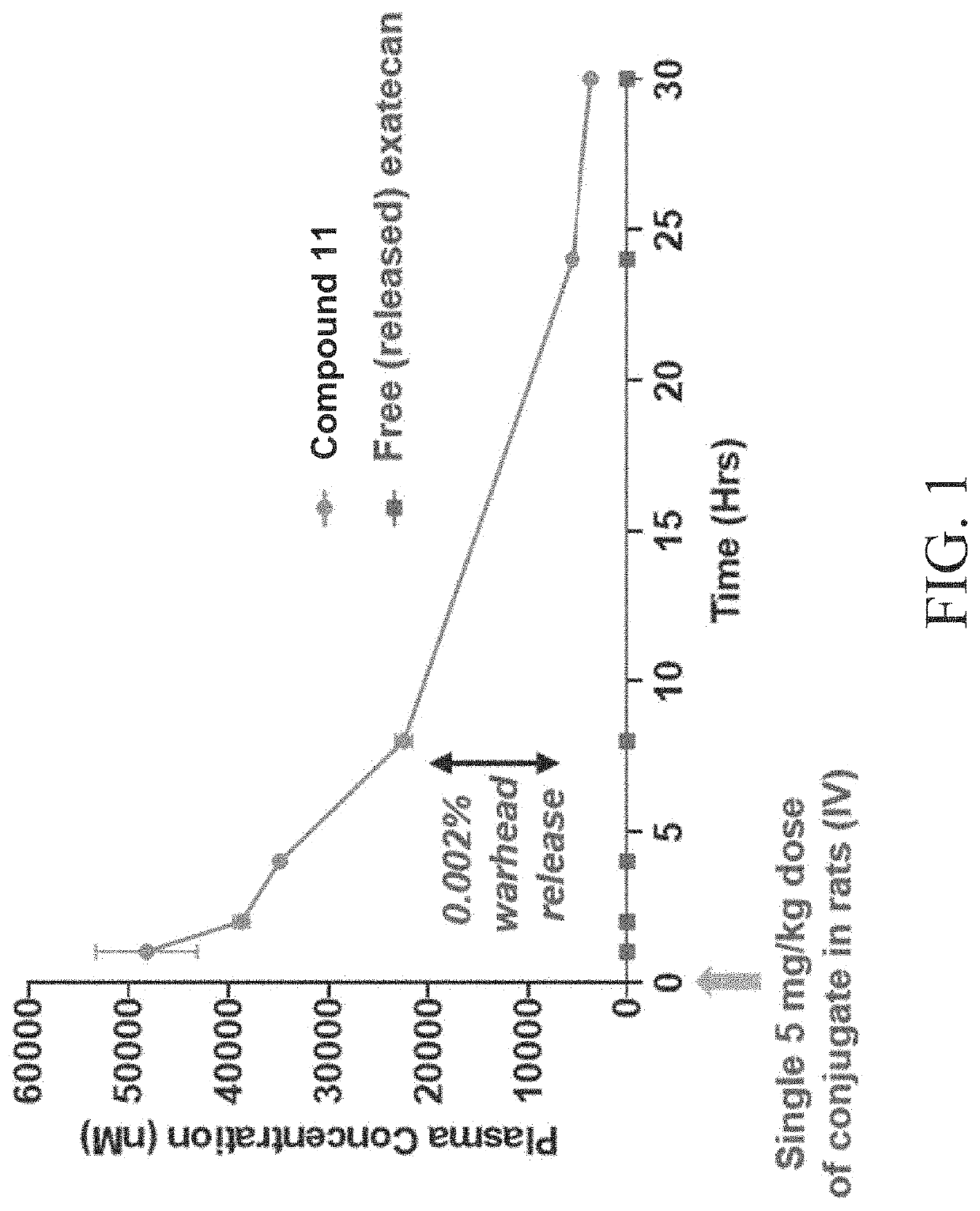
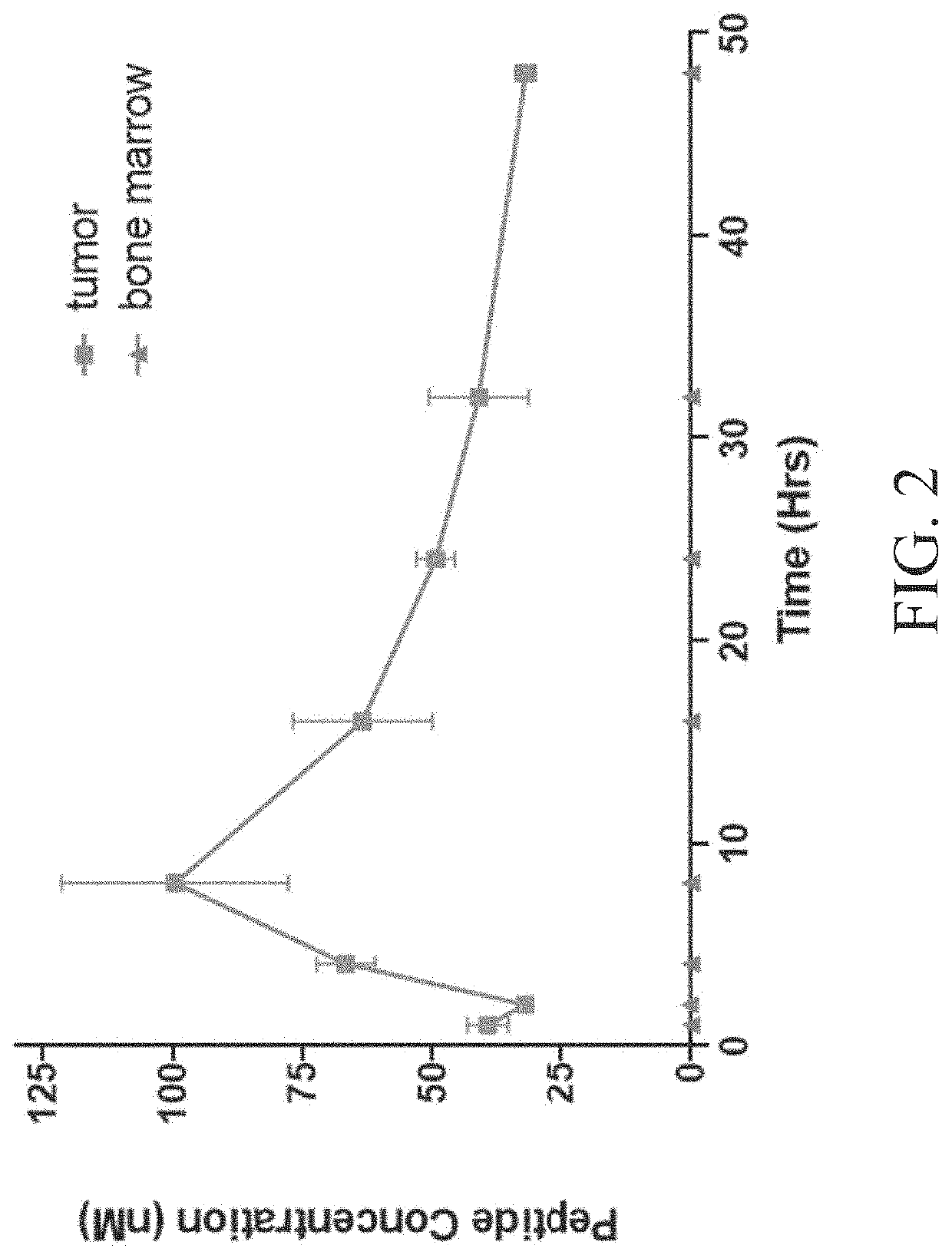
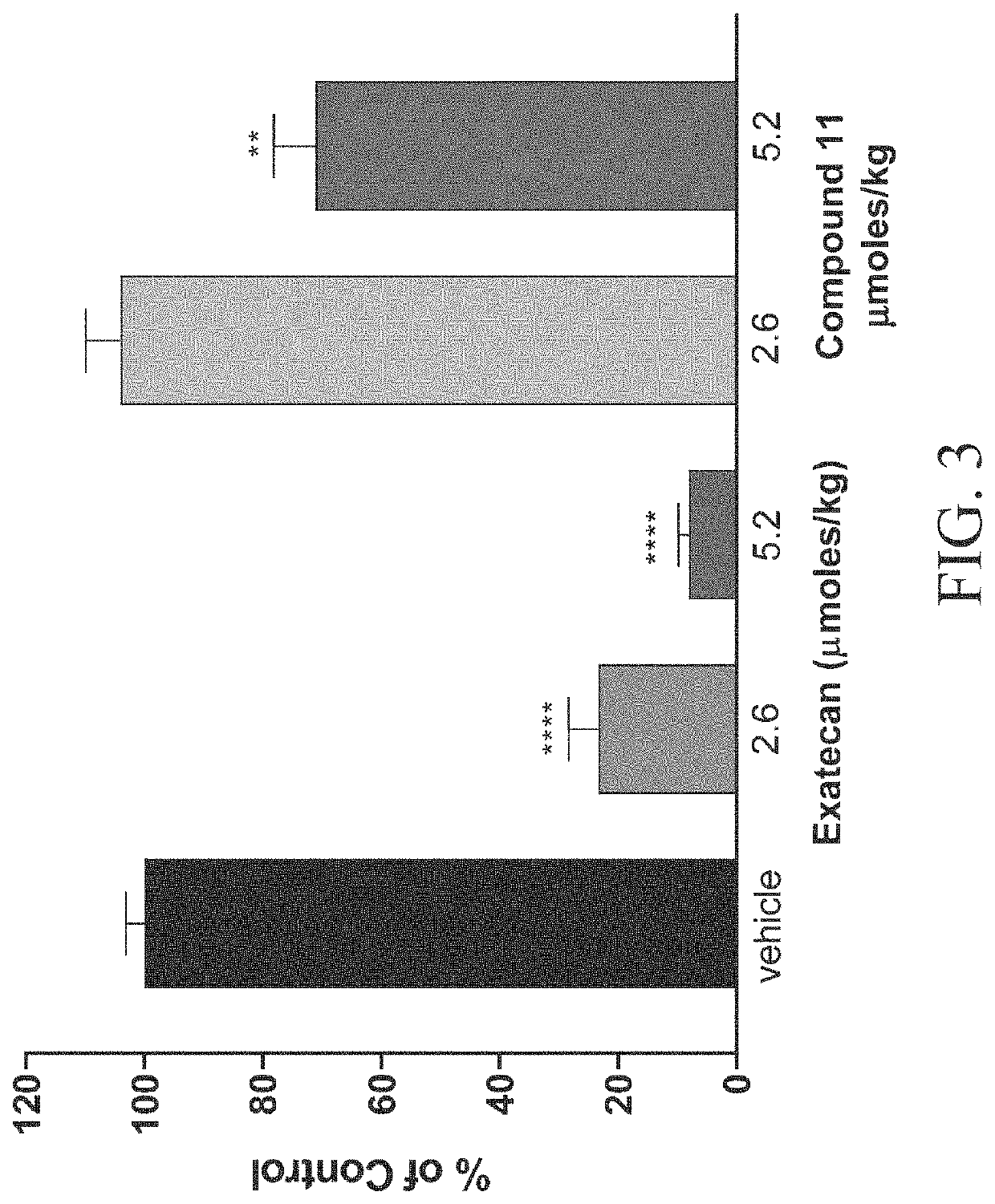
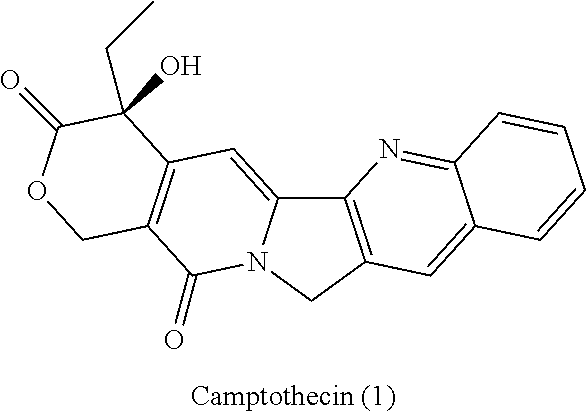
Patents
Literature
71 results about "Topoisomerase-I Inhibitor" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
Any substance that inhibits topoisomerase-I, a topoisomerase that relieves torsional stress in a DNA molecule by cutting only one strand of the DNA double helix. Inhibition of topoisomerase-I causes DNA damage, inhibition of DNA replication, and apoptosis.
Methods and compositions for modulating cell proliferation and cell death
InactiveUS6599912B1Enhance in vitroImprove in vivo activityBiocidePeptide/protein ingredientsAnticarcinogenTopoisomerase-II Inhibitor
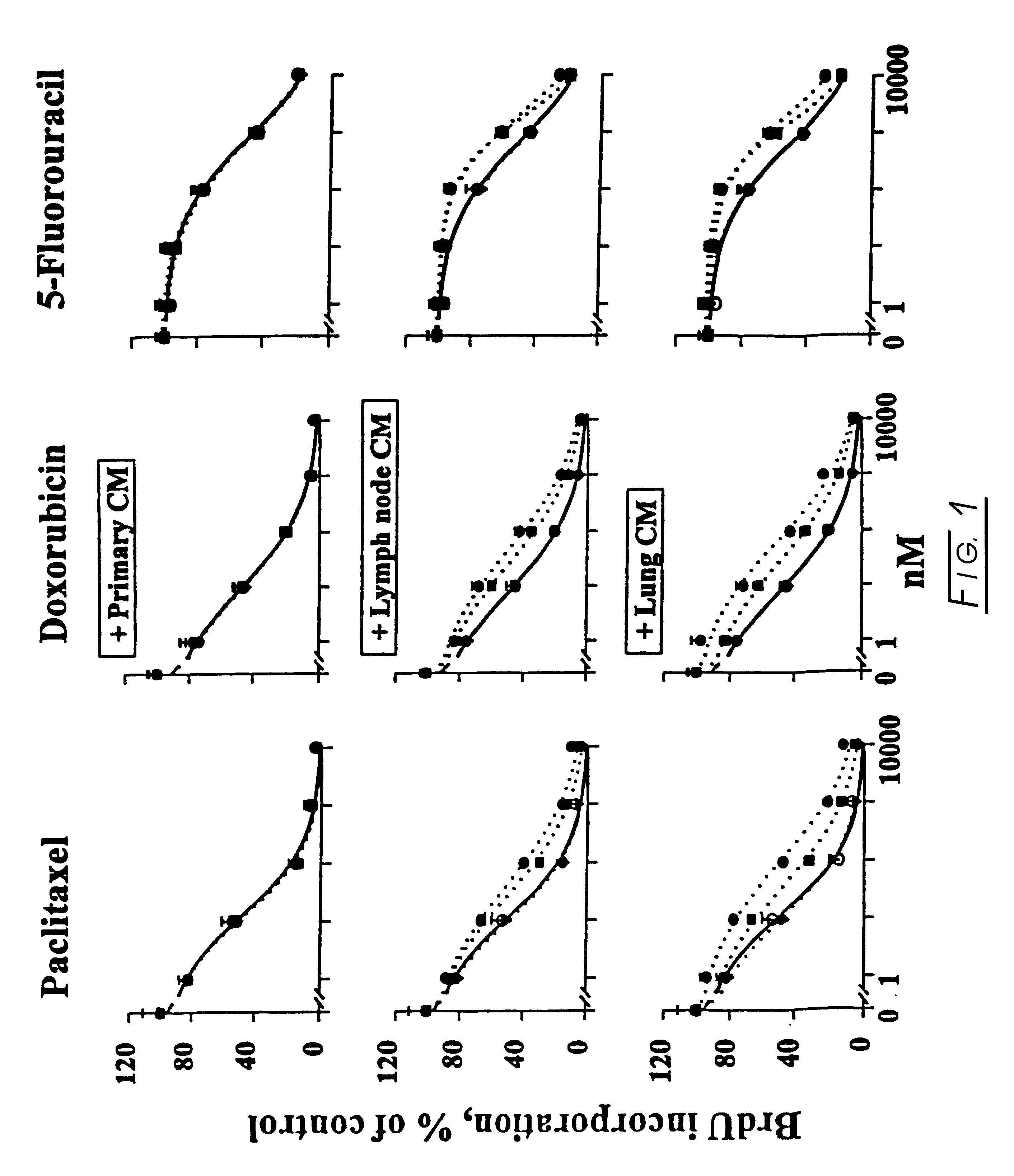
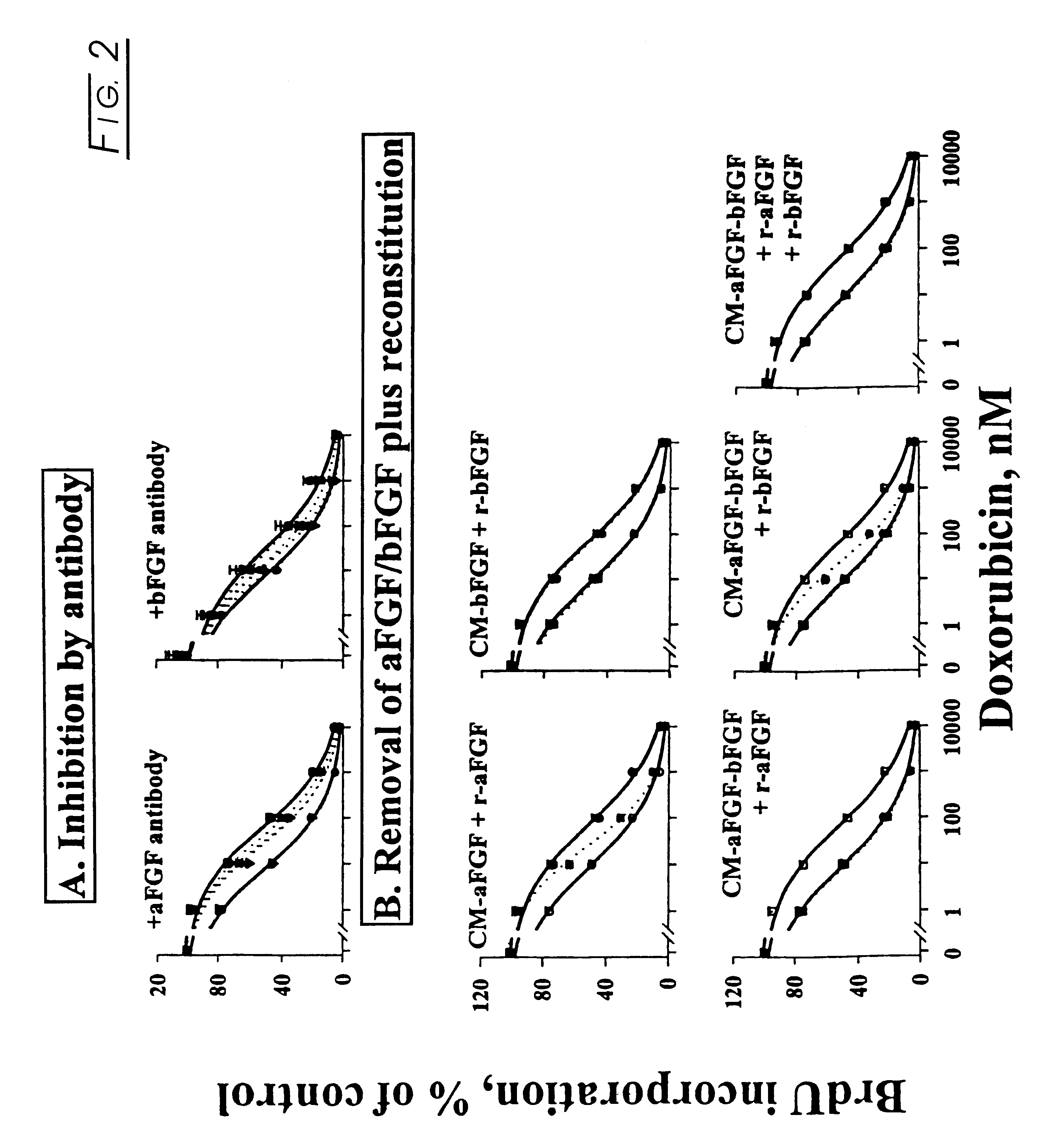
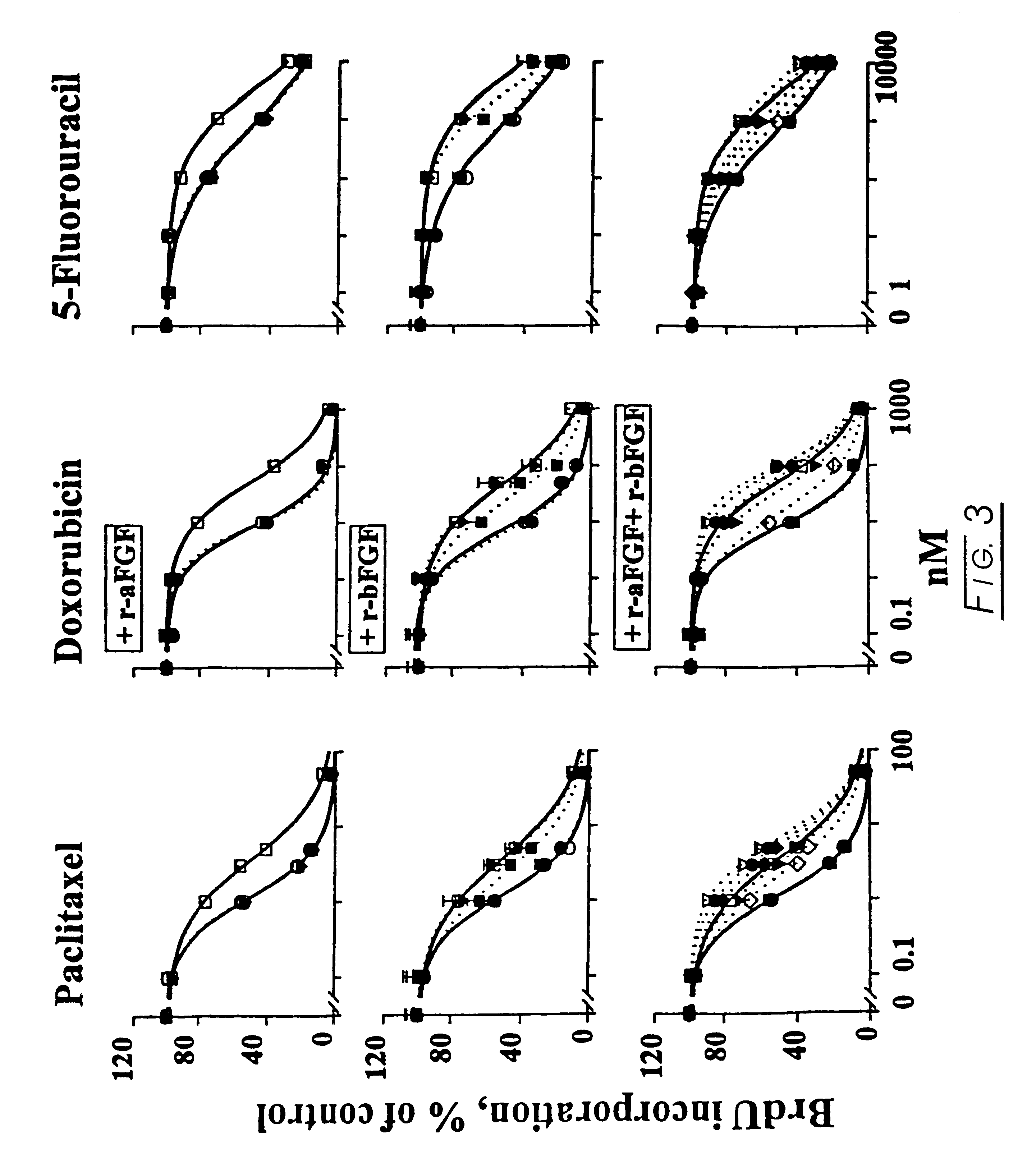
Methods and compositions for modulating the FGF effect on the sensitivity of malignant and normal cells to anticancer agents are provided. In particular, methods and compositions for inhibiting FGF-induced resistance to a broad spectrum of anticancer agents in solid and soft-tissue tumors, metastatic lesions, leukemia and lymphoma are provided. Preferably, the compositions include at least one FGF inhibitor in combination with a cytotoxic agents, e.g., antimicrotubule agents, topoisomerase I inhibitors, topoisomerase II inhibitors, antimetabolites, mitotic inhibitors, alkylating agents, intercalating agents, agents capable of interfering with a signal transduction pathway (e.g., g., a protein kinase C inhibitor, e.g., an anti-hormone, e.g., an antibody against growth factor receptors), an agent that promotes apoptosis and / or necrosis, and interferon, an interleukin, a tumor necrosis factor, and radiation. In other embodiments, methods and composition for protecting a cell in a subject, from one or more of killing, inhibition of growth or division or other damage caused, e.g., by a cytotoxic agent, are provided. Preferably, the method includes: administering, to the subject, an effective amount of at least one FGF agonist, thereby treating the cell, e.g., protecting or reducing the damage to the dividing cell from said cytotoxic agent.
Owner:AU JESSIE L S +1
Novel Concomitant Use of Sulfonamide Compound with Anti-Cancer Agent
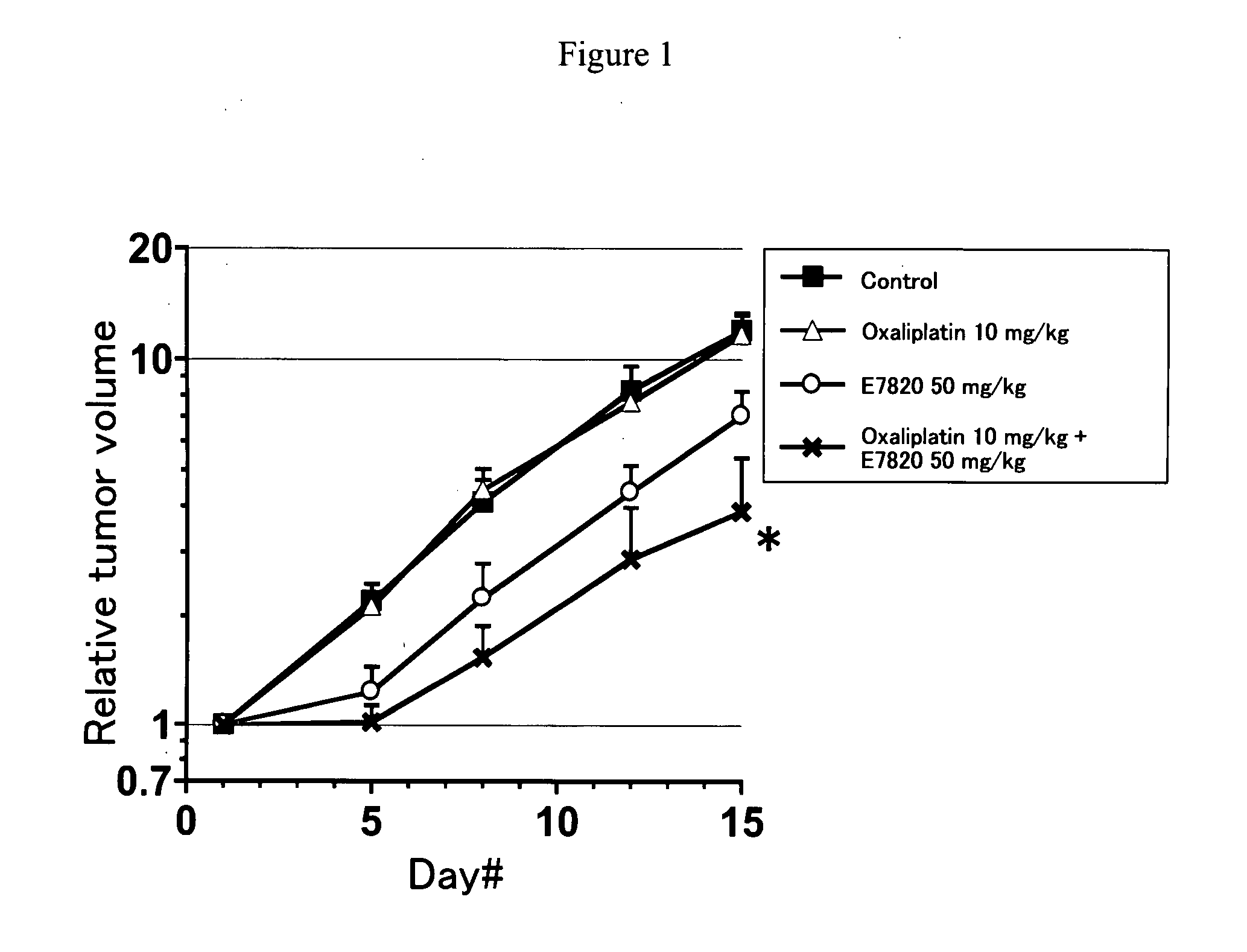
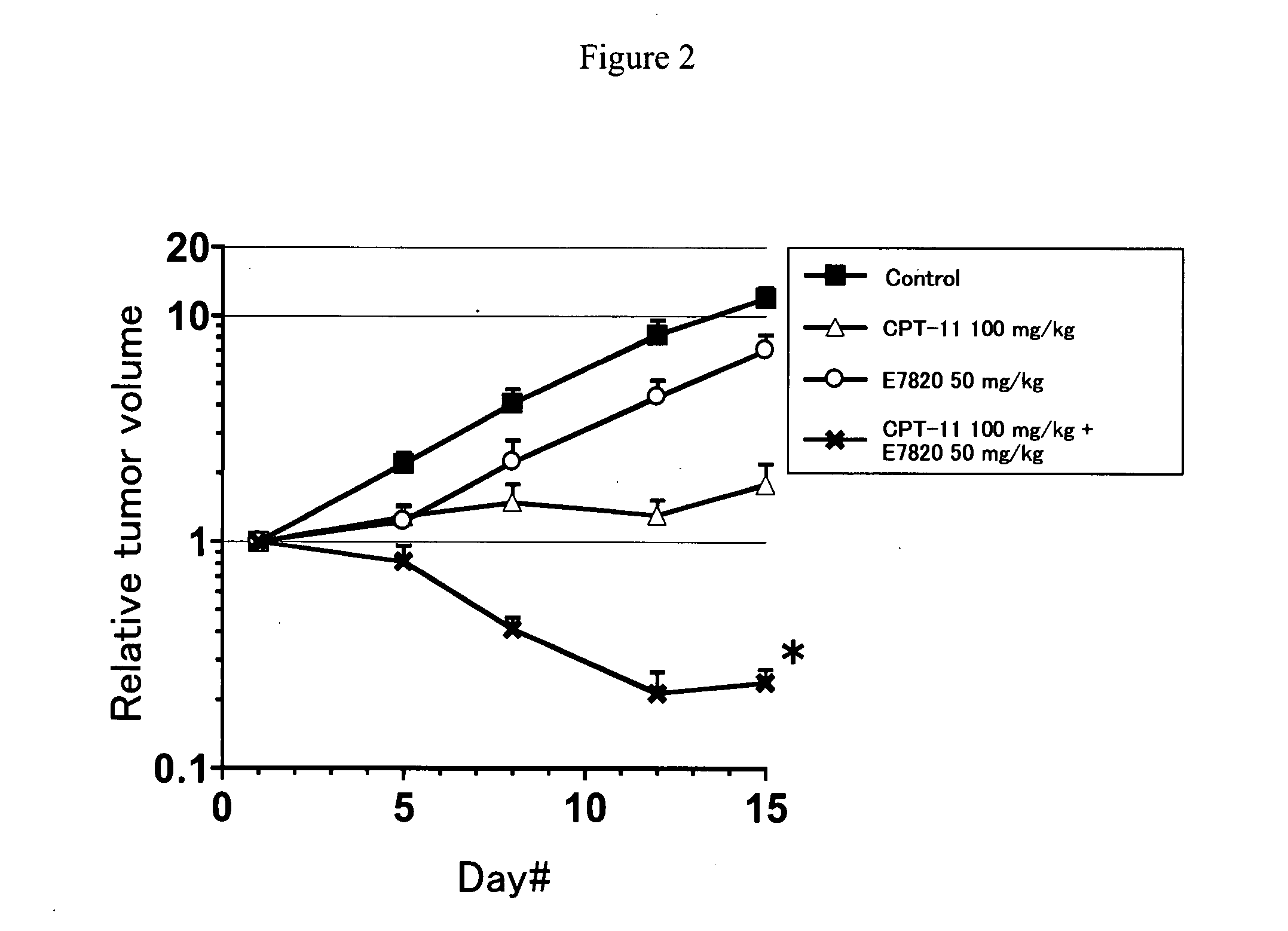
InactiveUS20090047365A1Improve anti-tumor activityGood activity angiogenesis inhibiting activityHeavy metal active ingredientsBiocidePlatinum complexTopoisomerase-I Inhibitor
The present invention relates to a pharmaceutical composition, a kit, a method of treating cancer and / or a method of inhibiting angiogenesis comprising a sulfonamide compound in combination with a platinum complex, a DNA-topoisomerase I inhibitor, an antimetabolite, a microtubule inhibitor or an antibiotic.
Owner:EISIA R&D MANAGEMENT CO LTD
Method for predicating homologous recombination deficiency mechanism and method for predicating response of patients to cancer therapy
InactiveCN107287285AInnovativeOvercoming the pitfalls of inaccurate forecastsMicrobiological testing/measurementSequence analysisAbnormal tissue growthPolymerase L
The invention discloses a method for predicating a homologous recombination deficiency (HRD) mechanism and a method for predicating response of patients to cancer therapy and relates to the field of biological information predication. The method comprises the step of judging whether a tumor sample has homologous recombination deficiency or not according to one or more comprehensive values in a large-segment INDEL (Insertion / Deletion) fraction, a copy number variation fraction and a tumor mutation load fraction, wherein the comprehensive values can also comprise a loss of heterozygosity variation fraction. By adopting the method disclosed by the invention, predication of a chromosome large-segment structure, a chromosome gene type number, a chromosome gene copy number, a chromosome variation interval and abnormal loss of heterozygosity and chromosome telomeric imbalance is realized, so that an evaluation range is more complete and HRD can be accurately predicated; the comprehensive values also can be used for determining whether the patients have response to a therapeutic regimen containing one or more of a PARP (Poly Adenosine Diphosphate Ribose Polymerase) inhibitor, an DNA (Deoxyribonucleic Acid) injury inhibitor, a topoisomerase II / II+inhibitor, a topoisomerase I inhibitor and radiotherapy; the method is simple and has wide general applicability.
Owner:SHANGHAI ORIGIMED CO LTD
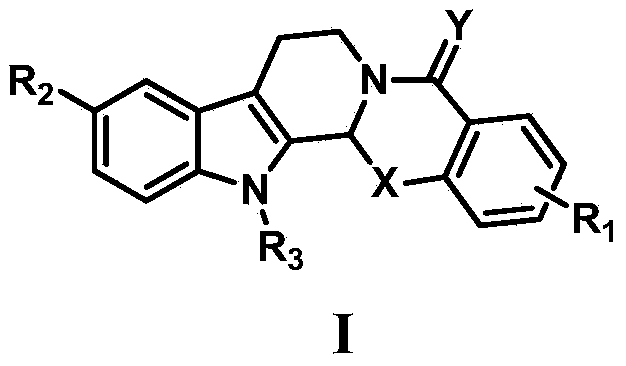
Oxa- or thio-evodiamine anti-tumor derivatives and preparation method thereof
ActiveCN103992336AGood antitumor activityOrganic active ingredientsOrganic chemistryDNA underwindingTopoisomerase-I Inhibitor
The invention relates to the technical field of medicines and in particular relates to oxa- or thio-evodiamine anti-tumor derivatives as well as a preparation method of the derivatives and application of the derivatives in preparation of topoisomerase inhibitors and anti-tumor drugs. The oxa- or thio-evodiamine anti-tumor derivatives are newly discovered topoisomerase I inhibitors with brand-new structures and have remarkable anti-tumor activity, and the structure of a compound is as shown in a general formula I.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
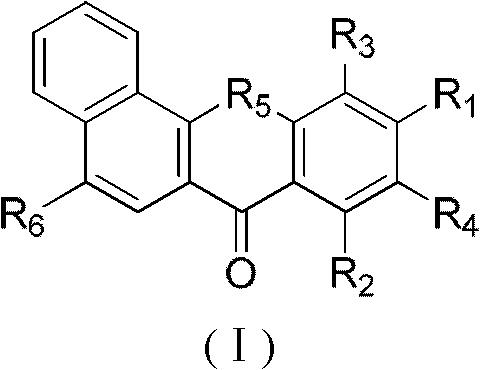
Substituted benzoxanthone type compound and application thereof
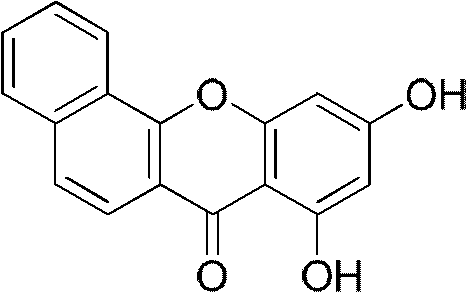
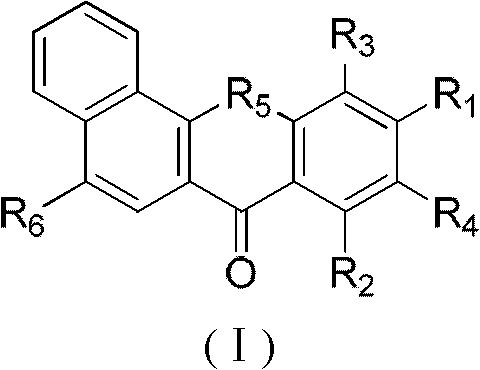
InactiveCN102070595AGood antitumor activityHigh topoisomerase I contentOrganic active ingredientsAntimycoticsHypertension medicationsAntifungal
The invention relates to the technical field of medicine, in particular to a new benzoxanthone type compound and an application thereof in pharmacy. Benzoxanthone type derivatives are one type of topoisomerase I inhibitors in new structure, which are found in recent years, and have obvious cell proliferation activity. The invention provides the new benzoxanthone type compound and pharmaceutical salts thereof, and the structure of the compound is as shown in the general formula (I). The invention further provides the application of the benzoxanthone type compound and the pharmaceutical salts thereof in the preparation of the topoisomerase inhibitors, anti-tumor medicaments, antifungal medicaments, antiviral medicaments, anti-hypertensive medicaments or anti-thrombotic medicaments.
Owner:SECOND MILITARY MEDICAL UNIV OF THE PEOPLES LIBERATION ARMY
Therapeutic combination comprising a cdc7 inhibitor and an Anti-neoplastic agent
ActiveUS20120276093A1Less side effectsStrong synergyHeavy metal active ingredientsBiocideCell Surface ProteinsAntimitotic Agent
The present invention provides a therapeutic combination comprising (a) a compound of formula (I) as set forth in the specification and (b) one or more antineoplastic agents selected from the group consisting of an alkylating or alkylating-like agent, an antimetabolite agent, a topoisomerase I inhibitor, a topoisomerase 11 inhibitor, an inhibitor of a growth factor or of a growth factor receptor, an antimitotic agent, a proteasome inhibitor, an inhibitor of an anti-apoptotic protein and an antibody directed against a cell surface protein, wherein the active ingredients are present in each case in lice form or in the form of a pharmaceutically acceptable salt or any hydrate thereof.
Owner:NERVIANO MEDICAL SERVICES SRL
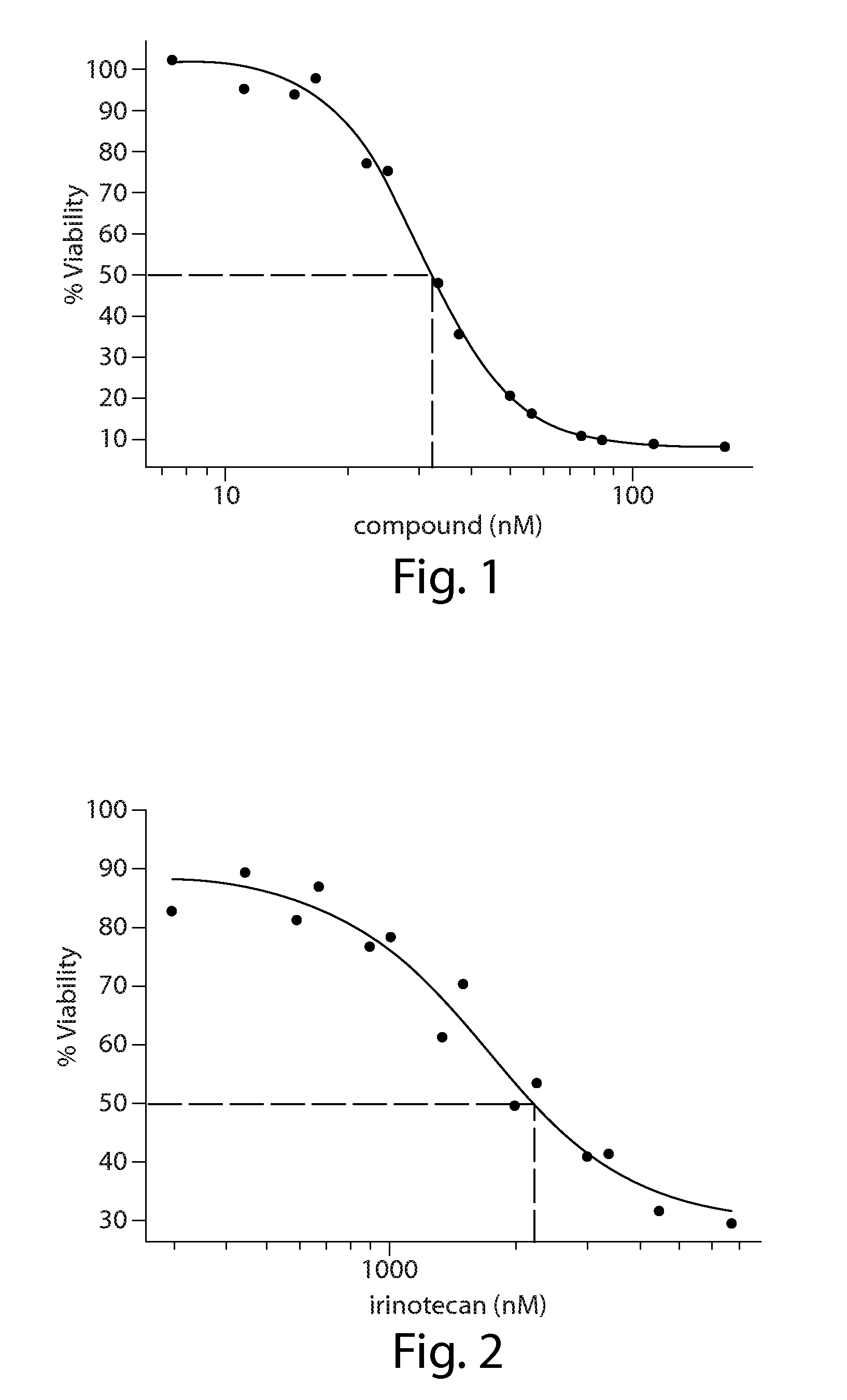
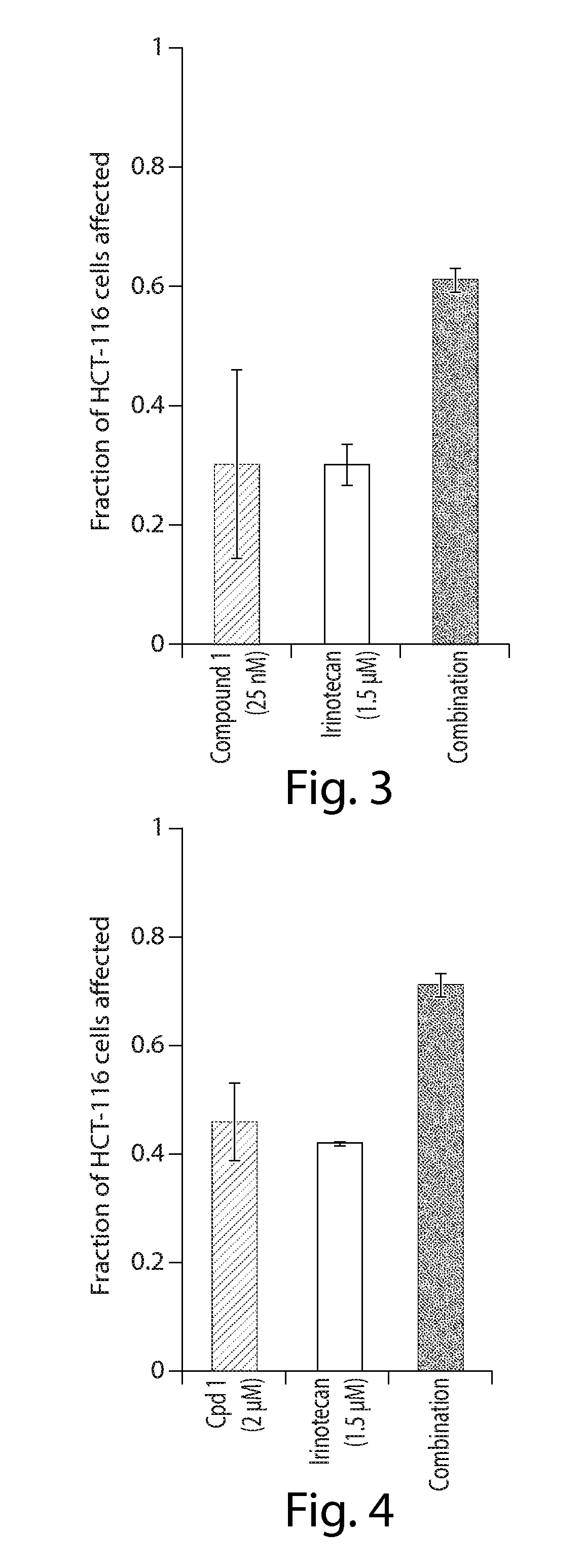
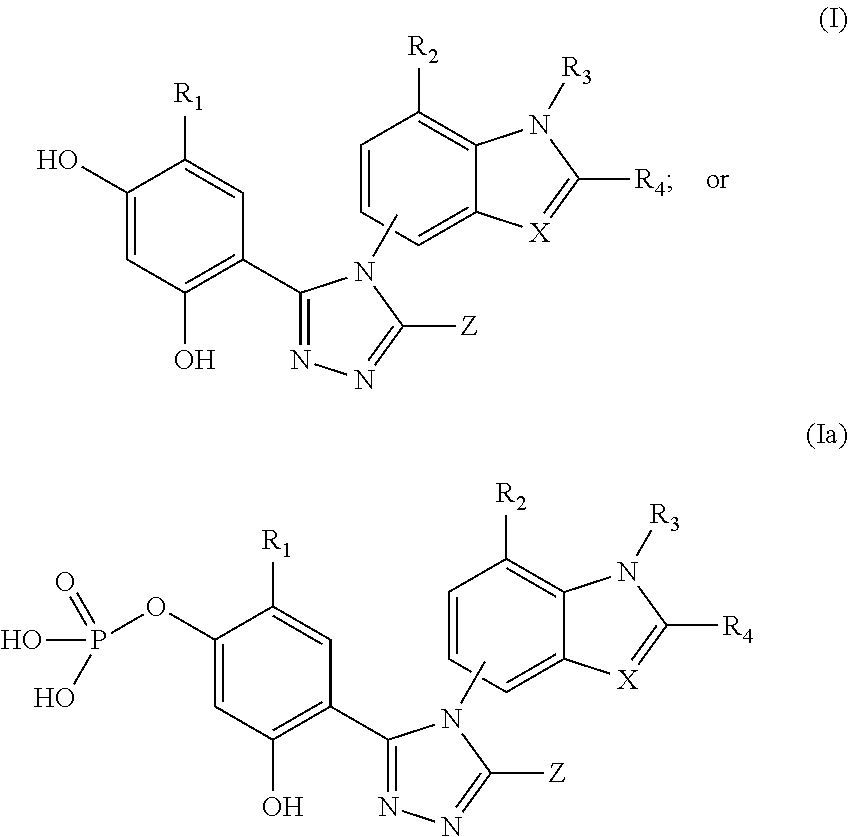
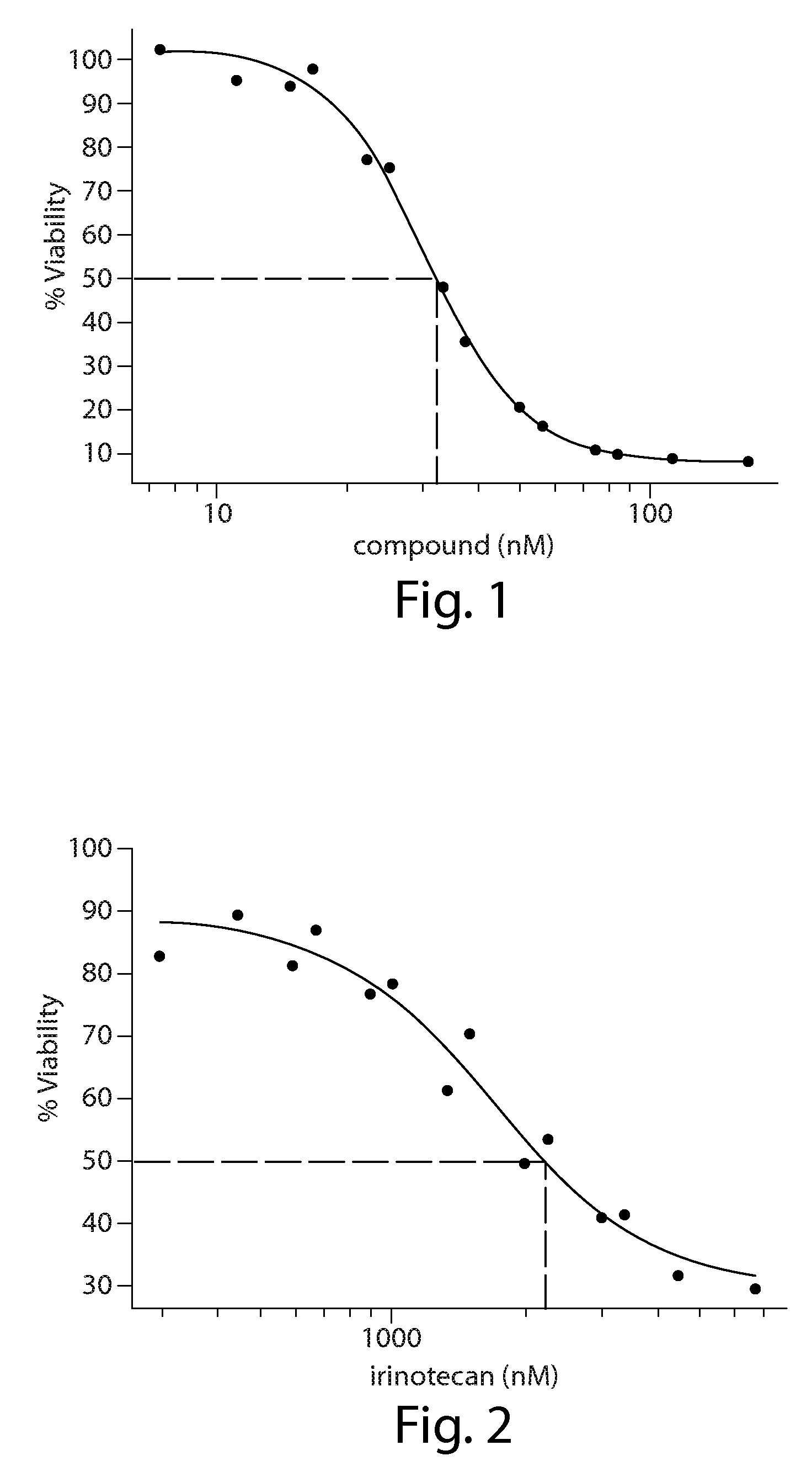
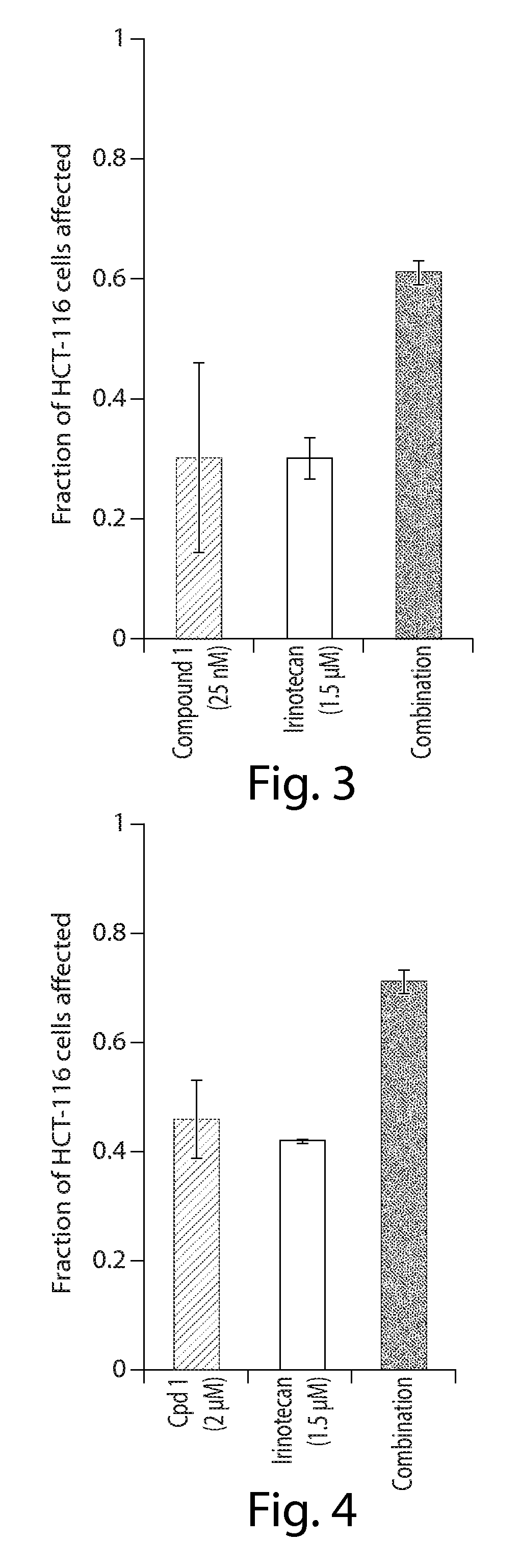
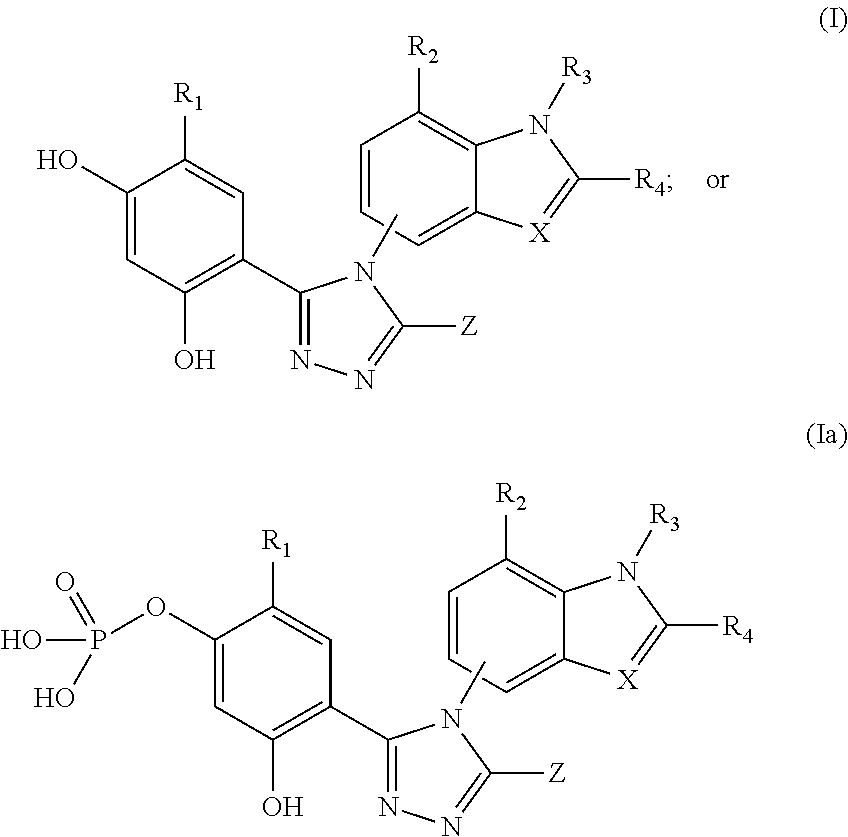
Cancer therapy using a combination of hsp90 inhibitors with topoisomerase i inhibitors
ActiveUS20140255348A1Increasing side effect profileSurprising biological activityBiocidePeptide/protein ingredientsDiseaseDNA underwinding
A pharmaceutical combination comprising a topoisomerase I inhibitor, and an Hsp90 inhibitor according to the following formulae (I) (Ia) a tautomer, or a pharmaceutically acceptable salt thereof, wherein the variables in the structural formulae are defined herein. Also provided is a method for treating a proliferative disorder in a subject in need thereof, using the pharmaceutical combination described herein.
Owner:SYNTA PHARMA CORP
Treatment Of RB-Negative Tumors Using Topoisomerase Inhibitors In Combination With Cyclin Dependent Kinase 4/6 Inhibitors
This invention is in the area of improved therapeutic combinations for and methods of treating selected retinoblastoma (Rb)-negative cancers and Rb-negative abnormal cellular proliferative disorders using particular topoisomerase inhibitors and specific cyclin-dependent kinase 4 / 6 (CDK4 / 6) inhibitors. In one aspect, the improved treatment of select Rb-negative cancers is disclosed using specific compounds disclosed herein in combination with a topoisomerase I inhibitor.
Owner:G1 THERAPEUTICS INC
Combination of a Cox-2 inhibitor and a DNA topoisomerase I inhibitor for treatment of neoplasia
InactiveUS20050187172A1Selective in their physiological impactPrevention and treatment of neoplasiaBiocideCarbohydrate active ingredientsTopoisomerase-I InhibitorCOX-2 inhibitor
The present invention provides combinations of a Cox-2 inhibitor and a DNA topoisomerase inhibitor and methods of use thereof for preventing and / or treating neoplasia or or a neoplasia-related disorder in a subject.
Owner:PFIZER INC
Azaindenoisoquinoline topoisomerase i inhibitors
ActiveUS20140018360A1Increase distanceProlonged covalent attachmentBiocideOrganic chemistryDNA underwindingIsoquinoline
The invention described herein pertains to substituted azaindenoisoquinoline compounds, in particular 7-, 8-, 9-, and 10-azaindenoisoquinoline compounds, which are inhibitors of topoisomerase I, processes and intermediates for their syntheses, pharmaceutical compositions of the compounds, and methods of using them in the treatment of cancer.
Owner:PURDUE RES FOUND INC
Azaindenoisoquinoline topoisomerase i inhibitors
ActiveUS20140187547A1Good chemical stabilityGood water solubilityBiocideOrganic chemistryDNA underwindingIsoquinoline
The invention described herein pertains to substituted azaindenoisoquinoline compounds, in particular 7-, 8-, 9-, and 10-azaindenoisoquinoline compounds, which are inhibitors of topoisomerase I, processes and intermediates for their syntheses, pharmaceutical compositions of the compounds, and methods of using them in the treatment of cancer.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH +1
Novel topoisomerase I inhibitor and pharmaceutical compositions thereof as well as preparation methods and applications of novel topoisomerase I inhibitor and pharmaceutical compositions thereof
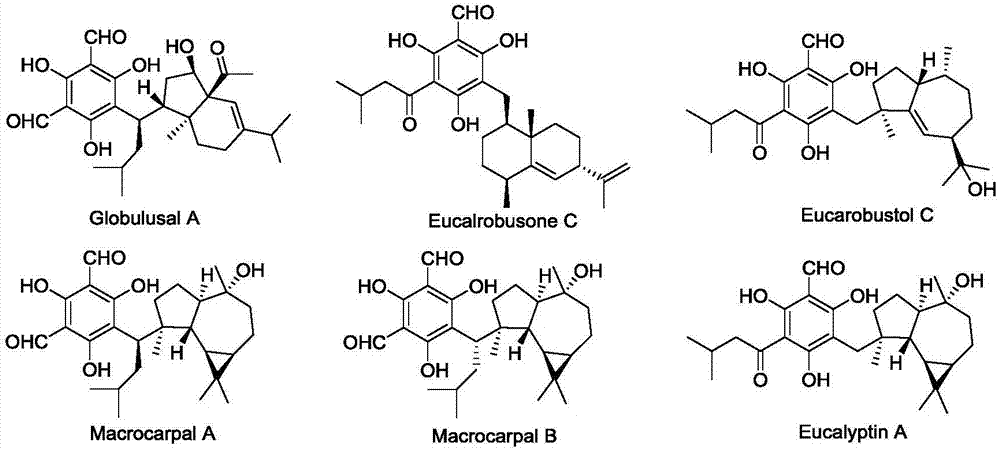
ActiveCN107986951AOrganic chemistry methodsKetone active ingredientsFunctional healthEucalyptus globulus
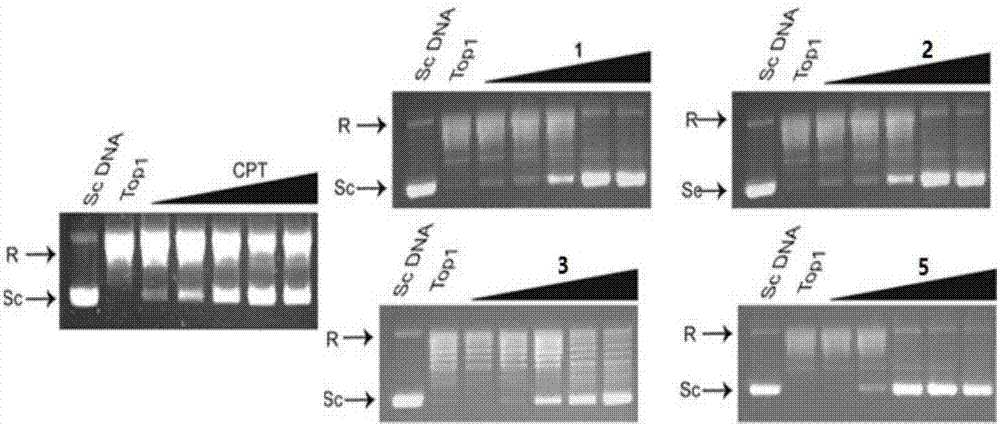
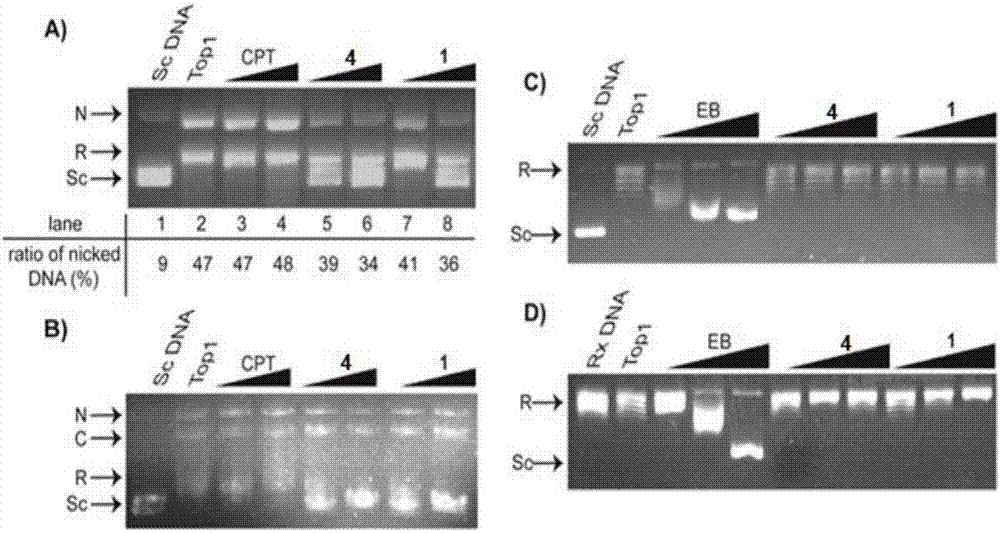
The invention relates to formyl phloroglucinol hetero terpenoids separated out from fruit of eucalyptus globulus, pharmaceutical compositions taking the formyl phloroglucinol hetero terpenoids as active components, preparation methods of the formyl phloroglucinol hetero terpenoids and the pharmaceutical compositions as well as applications of the formyl phloroglucinol hetero terpenoids and the pharmaceutical compositions in preparation of medicines for treating tumors and in preparation of functional health care products. The methods provided by the invention are easy in obtaining of raw materials, simple and easy to operate; biological experiments prove that the obtained compounds have better activity of inhibiting the growth of tumor cells; furthermore, the compounds 1, 2, 3 and 5 have aDNA inhibiting topoisomerase I (TOP1) activity similar to camptothecin, and the compounds 1 and 4 have a better activity of promoting cancer cell apoptosis.
Method for fast screening topoisomerase I inhibitor from natural product
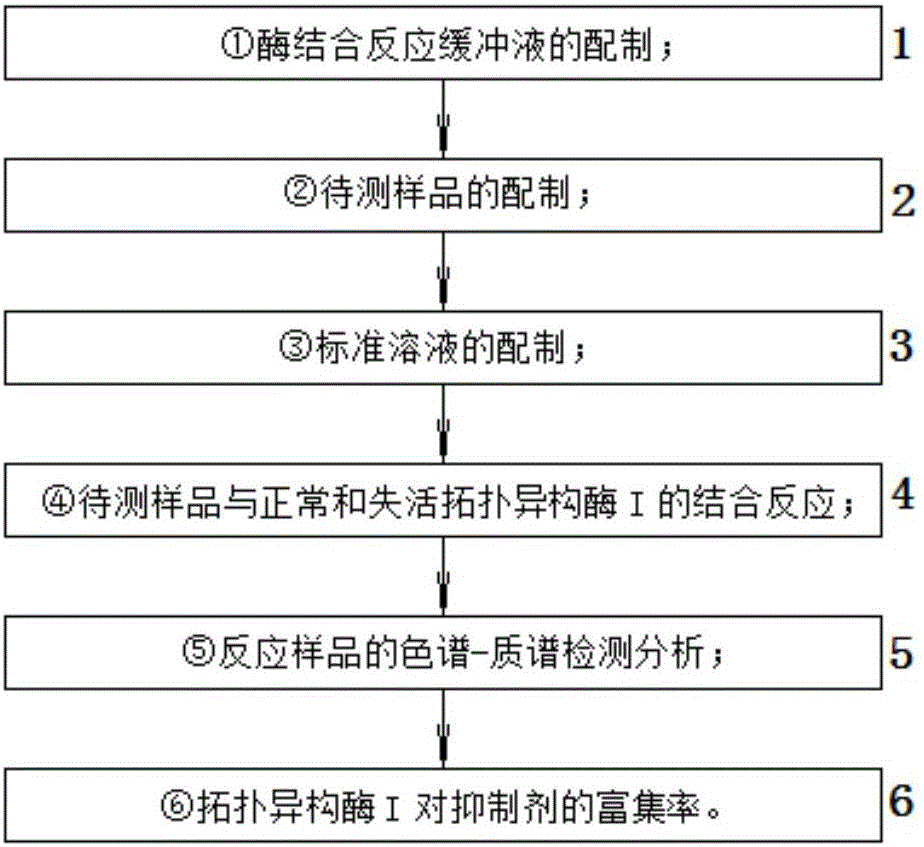
ActiveCN105717250AFast analysisStrong specificityComponent separationUltrafiltrationInformation quantity
The invention discloses a method for fast screening a topoisomerase I inhibitor from a natural product, and relates to the analysis field of natural products. The method comprises the following steps of: (1) preparation of an enzyme binding reaction buffer solution; (2) preparation of a to be detected sample; (3) preparation of a standard solution; (4) binding reaction of the to-be-detected sample and normal and inactive topoisomerase I; (5) chromatography-mass spectrometry detection analysis of reaction sample; and (6) enrichment ratio of the topoisomerase I to the inhibitor. The ultrafiltration membrane technology and the chromatography-mass spectrometry united technology united method are applied to the screening of the topoisomerase I inhibitor, the sample analysis speed is fast and the specificity is strong so as to conveniently recognize a medicine ligand combined with a biological target molecule; meanwhile, the sample analysis dosage is less, and the spectrogram information quantity is large so as to realize the fast screening of the lead compound, the method has great advantage on the aspect of researching mutual effect of medicine small molecule ligand and biological macromolecule receptor; the method is suitable for analyzing in-vitro enrichment ratio of the natural product extract or monomer compound to the topoisomerase I inhibitor.
Owner:WUHAN BOTANICAL GARDEN CHINESE ACAD OF SCI
Pharmaceutical composition comprising a campothecin derivative
InactiveUS20100166843A1Overcome instabilityOvercomes poor solubility problemBiocidePeptide/protein ingredientsMedicineTopoisomerase-I Inhibitor
The present invention relates to pharmaceutical compositions comprising a topoisomerase I inhibitor including, but not limited to, a camptothecin derivative.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
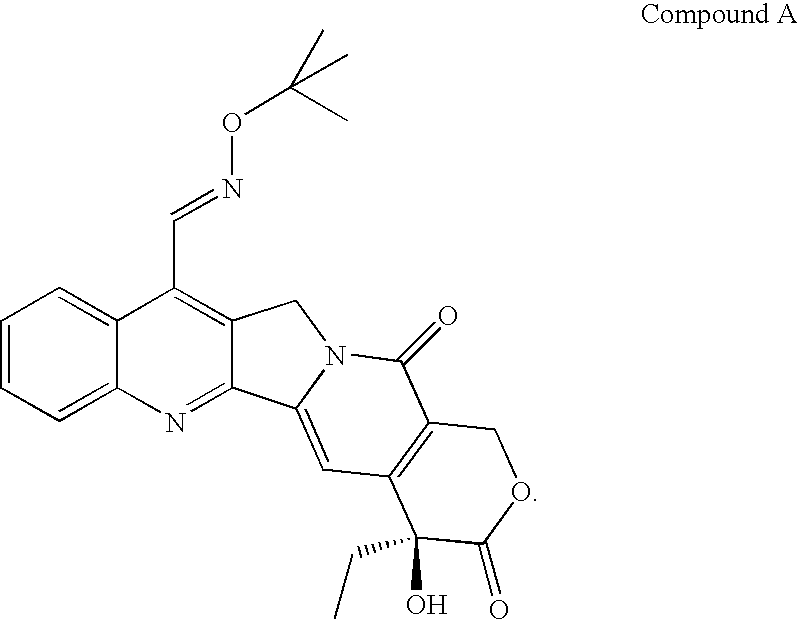
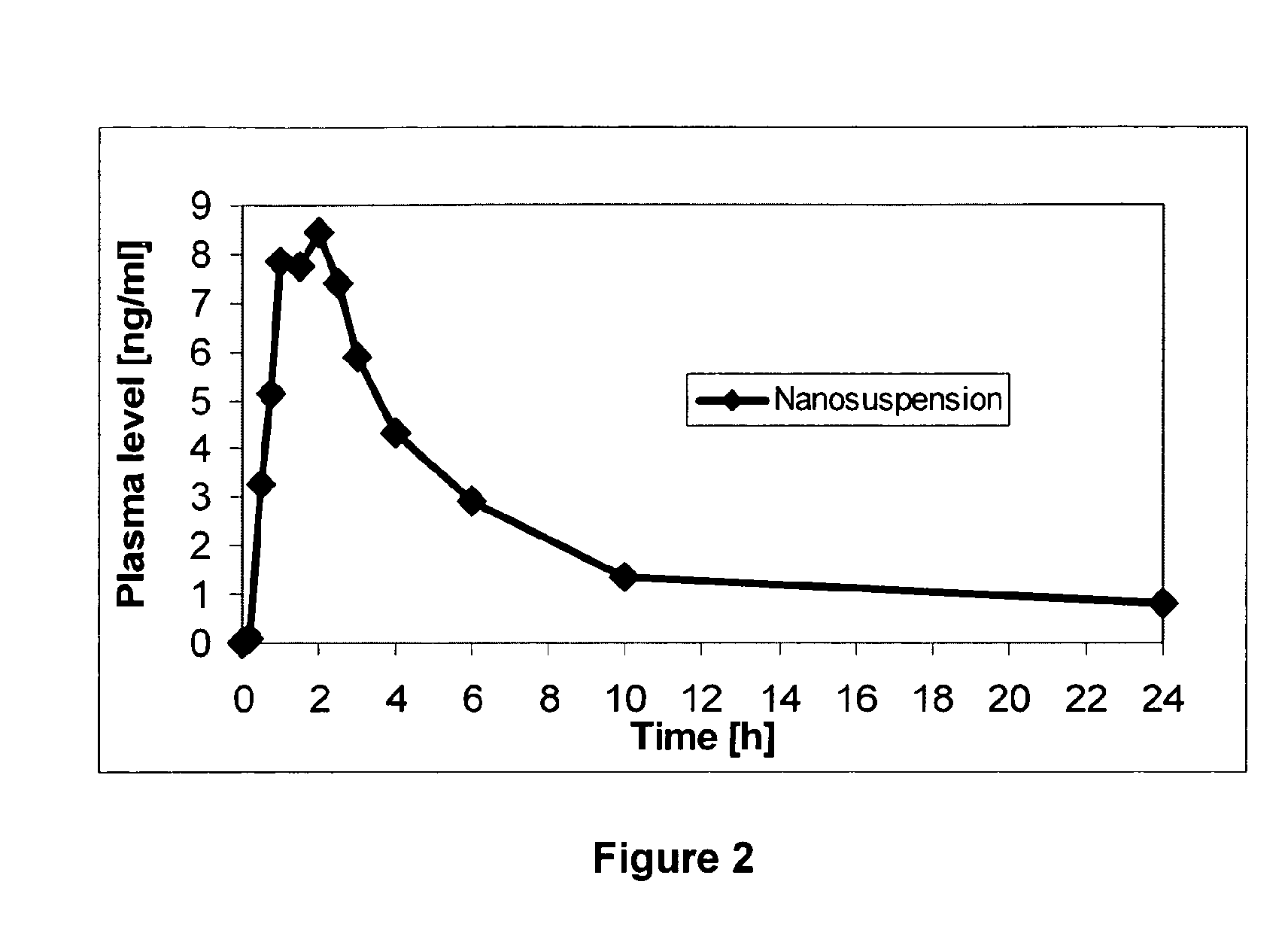
Formulations for 7- (T-Butoxy) Iminomethyl Camptothecin
The present invention relates to nanoparticulate compositions in which the active agent is a topoisomerase I inhibitor and pharmaceutical compositions comprising the nanoparticulate compositions that are useful for the treatment and prevention of proliferative diseases including cancer.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Pharmaceutical composition comprising a campothecin derivative
InactiveCN101652125AIncreased anti-tumor potencyExtend cycle timeOrganic active ingredientsAntineoplastic agentsTopoisomerase-I InhibitorMedicine
The present invention relates to pharmaceutical compositions comprising a topoisomerase I inhibitor including, but not limited to, a camptothecin derivative.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA
Treatment of rb-negative tumors using topoisomerase inhibitors in combination with cyclin dependent kinase 4/6 inhibitors
This invention is in the area of improved therapeutic combinations for and methods of treating selected retinoblastoma (Rb)-negative cancers and Rb-negative abnormal cellular proliferative disorders using particular topoisomerase inhibitors and specific cyclin-dependent kinase 4 / 6 (CDK4 / 6) inhibitors. In one aspect, the improved treatment of select Rb-negative cancers is disclosed using specific compounds disclosed herein in combination with a topoisomerase I inhibitor.
Owner:G1 THERAPEUTICS INC
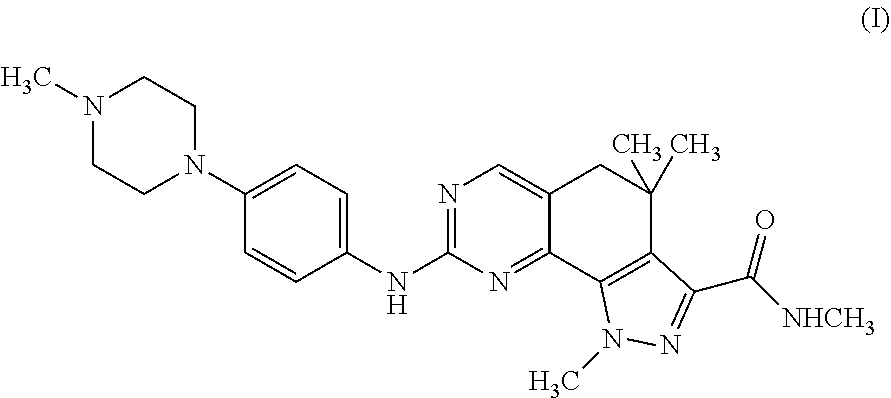
Esters in position 20 of camptothecins
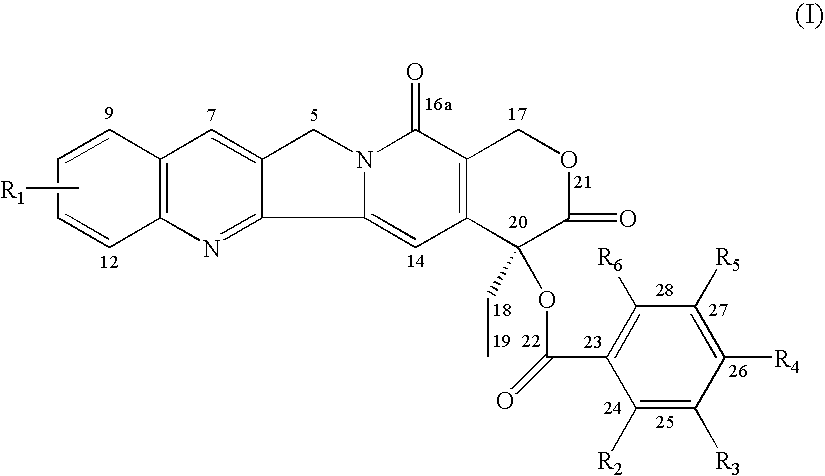
Formula (I) compounds are described: (I) where the groups are as defined in the description here below, the racemic mixtures, their individual enantiomers, their individual diastereoisomers, their mixtures, and their pharmaceutically acceptable salts. Said compounds are topoisomerase I inhibitors.
Owner:SIGMA TAU IND FARMACEUTICHE RIUNITE SPA +1
Predictive marker for topoisomerase I inhibitors
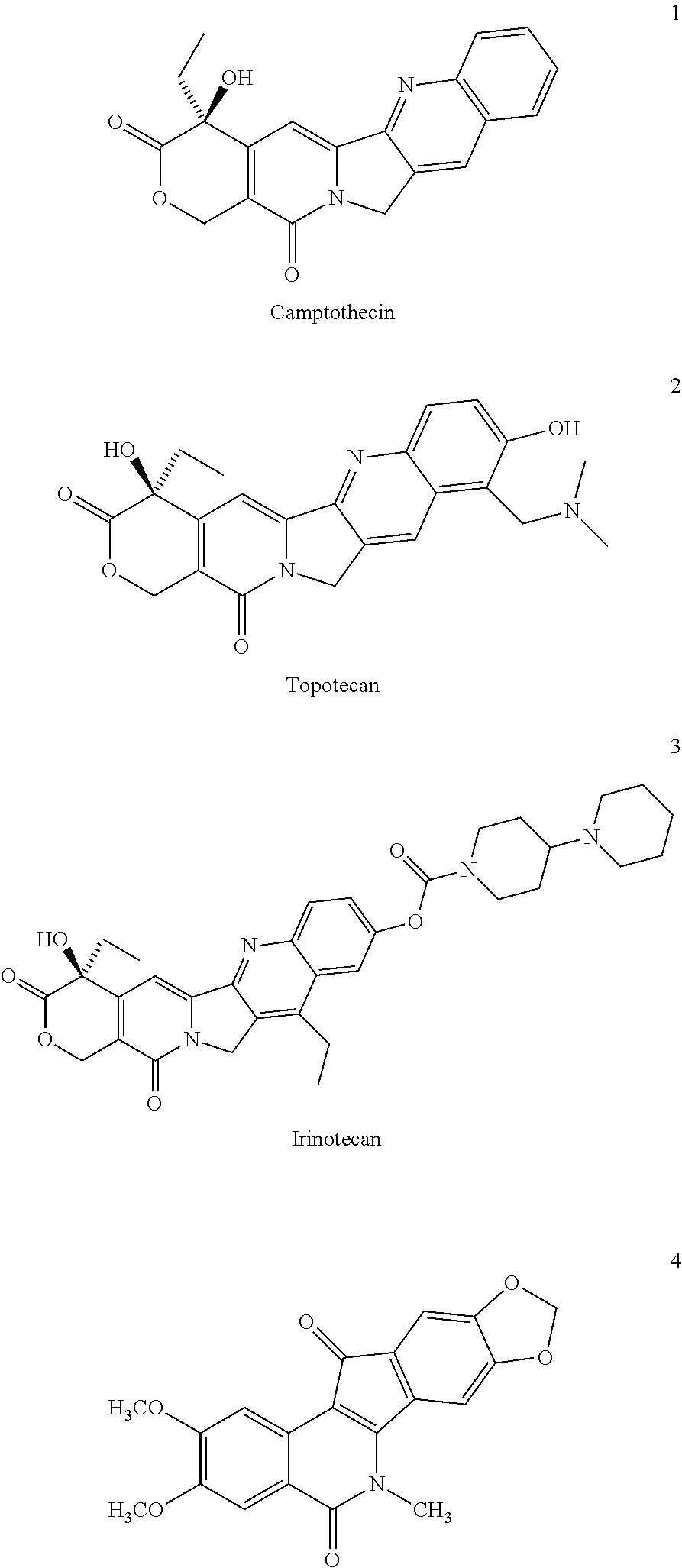
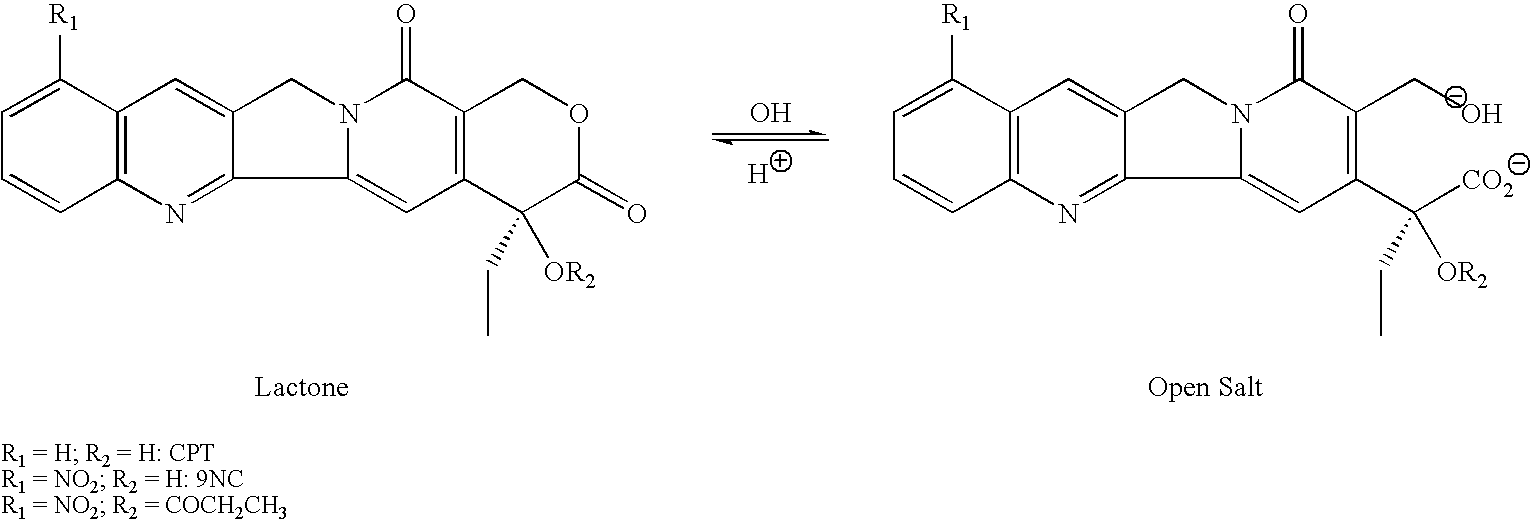
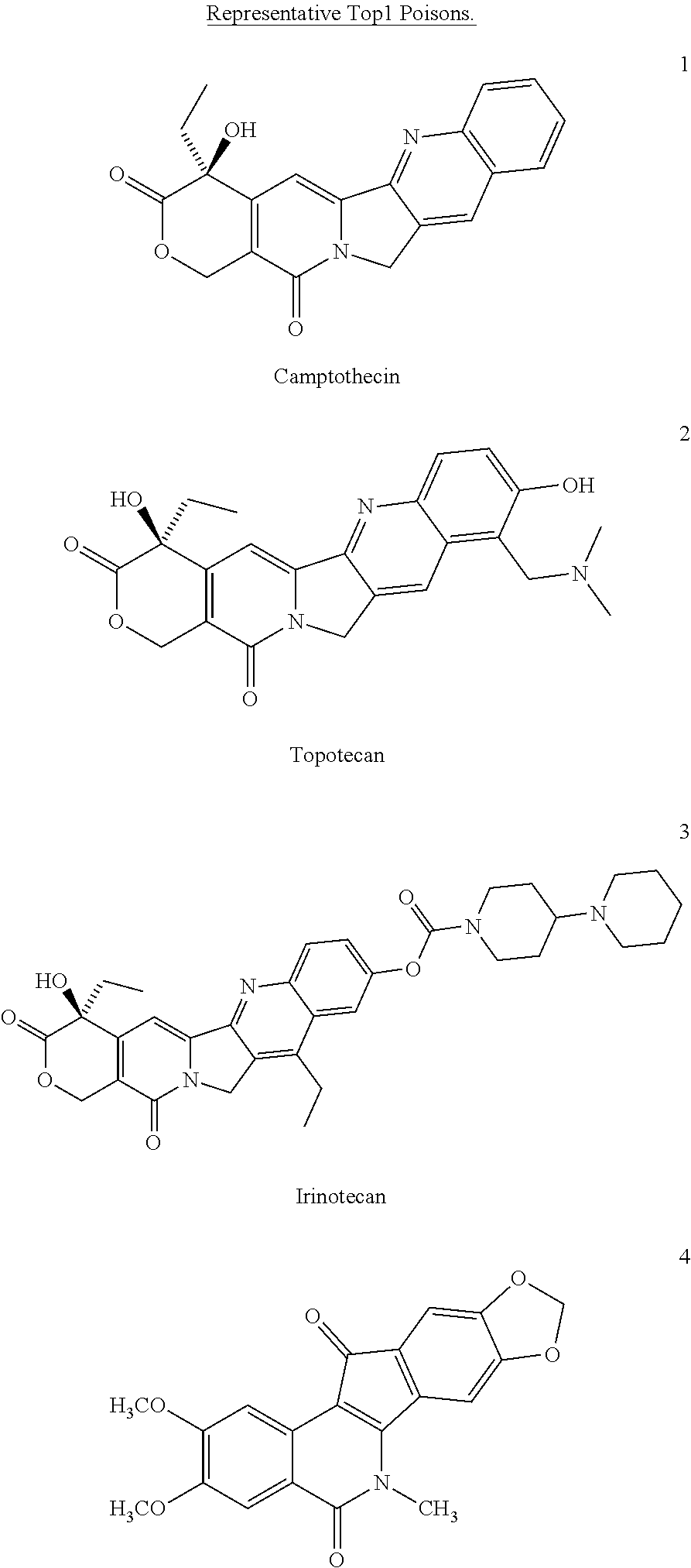
ActiveUS8993309B2Organic active ingredientsBioreactor/fermenter combinationsCancer preventionPredictive marker
The present invention generally relates to the fields of cancer therapy and cancer prevention. More particularly, the present invention generally relates to a diagnostic marker for predicting the efficacy of topoisomerase I (topo I) inhibitors in the treatment of cancers. More specifically, the present invention relates to methods, machines, computer systems, computable readable media and kits which can be used to identify and determine the effectiveness of topoisomerase I (topo I) inhibitors in the treatment of cancers, and in some embodiments, the level of sensitivity or resistance of a tumor cell to a topoisomerase I inhibitor, such as camptothecin (CPT), or CTP analogues such as topotecan and irinotecan and derivatives thereof. More specifically, the present invention related to methods, machines, computer systems, computable readable media and kits which can be used to determine the presence of phosphorylation of topoisomerase I polypeptide, in some embodiments phosphorylation at residue serine 10 (S10) of a topoisomerase I polypeptide, wherein the presence of phosphorylation, in particular the phosphorylation at serine 10 of a topoI polypeptide indicates a cancer is likely to be unresponsive to a topo I inhibitor, whereas the absence of phosphorylation, in particular, the absence of phosphorylation at residue serine 10 (S10) identifies a cancer is likely to be responsive to a topo I inhibitor. Other aspect of the present invention relate to phospho-serine 10 topoisomerase I antibodies and other protein binding moieties, and uses thereof.
Owner:BOSTON MEDICAL CENTER INC
Alcohol-, diol-, and carbohydrate-substituted indenoisoquinolines as topoisomerase i inhibitors
ActiveUS20160318946A1Potent Top inhibitory activityPotent antiproliferative activityOrganic chemistryAlcoholDNA underwinding
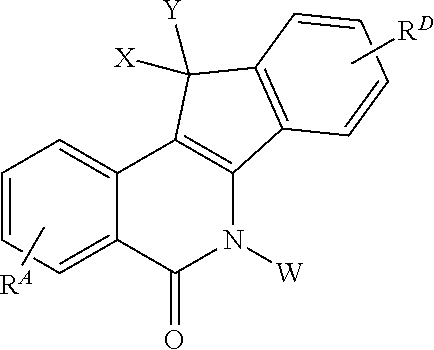
The invention described herein pertains to substituted indenoisoquinoline compounds as described herein, wherein RA, RD, W, X and Y are defined herein, pharmaceutical compositions and formulations comprising the indenoisoquinoline compounds, their synthesis, and methods for their use in the treatment and / or prevention of cancer.
Owner:PURDUE RES FOUND INC
Alternating treatment with topoisomerase I and topoisomerase II inhibitors
InactiveUS20040204435A1Simple methodBiocideCarbohydrate active ingredientsDNA underwindingTopoisomerase-I Inhibitor
A method to treat cancer is described which involves alternating treatments with a topoisomerase-I inhibitor and a topoisomerase-II inhibitor. Other aspects of the invention are further described.
Owner:THE STEHLIN FOUND FOR CANCER RES
Structures and methods for designing topoisomerase I inhibitors
This invention relates to crystalline structures of the topoisomerase I and their use in designing new anti-cancer agents anti-viral agents and anti-microbial agents.
Owner:EMERALD BIOSTRUCTURES
Therapeutic combination comprising a CDKS inhibitor and an antineoplastic agent
ActiveUS8518930B2Less side effectsStrong synergyBiocideCarbohydrate active ingredientsTopoisomerase-I InhibitorFree form
The present invention provides a therapeutic combination comprising (a) a compound of formula (I) as set forth in the specification and (b) one or more antineoplastic agents selected from the group consisting of alkylating or alkylating-like agents, antimetabolite agents and topoisomerase I inhibitors, wherein the active ingredients are present in each case in free form or in the form of a pharmaceutically acceptable salt or any hydrate thereof.
Owner:NERVIANO MEDICAL SERVICES SRL
Alcohol-, diol-, and carbohydrate-substituted indenoisoquinolines as topoisomerase I inhibitors
ActiveUS9328073B2Potent Top poisoning and antiproliferative activityInhibitory activityOrganic chemistryAntineoplastic agentsDNA underwindingAlcohol
Owner:PURDUE RES FOUND INC
Alcohol-, diol-, and carbohydrate-substituted indenoisoquinolines as topoisomerase I inhibitors
ActiveUS9682990B2Potent Top poisoning and antiproliferative activityInhibitory activityOrganic chemistryAlcoholDNA underwinding
The invention described herein pertains to substituted indenoisoquinoline compounds as described herein, wherein RA, RD, W, X and Y are defined herein, pharmaceutical compositions and formulations comprising the indenoisoquinoline compounds, their synthesis, and methods for their use in the treatment and / or prevention of cancer.
Owner:PURDUE RES FOUND INC
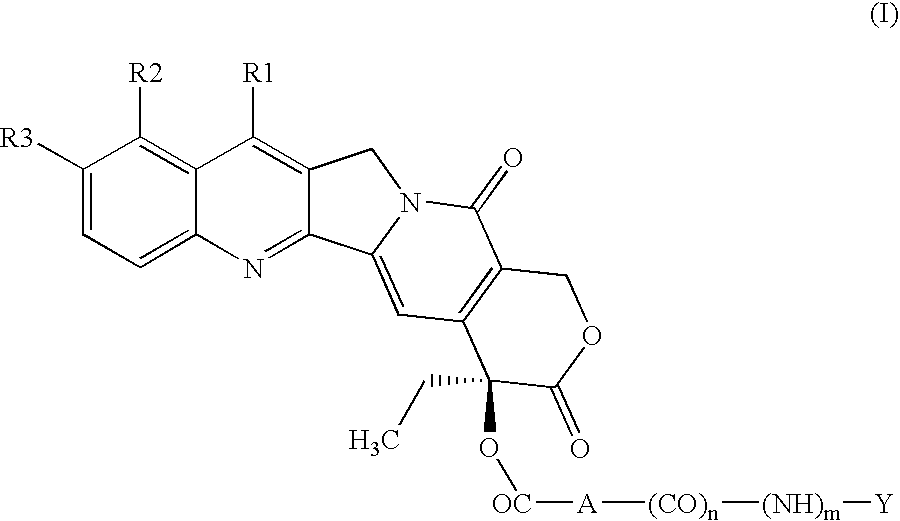
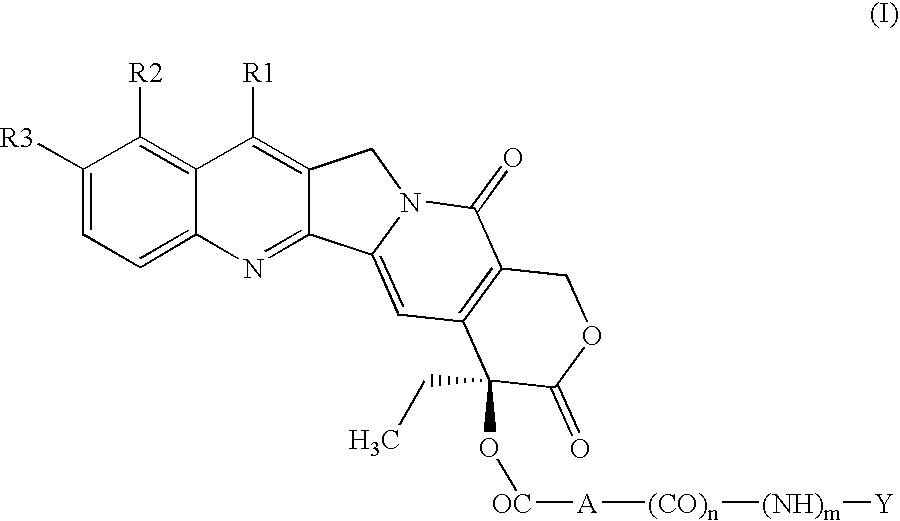
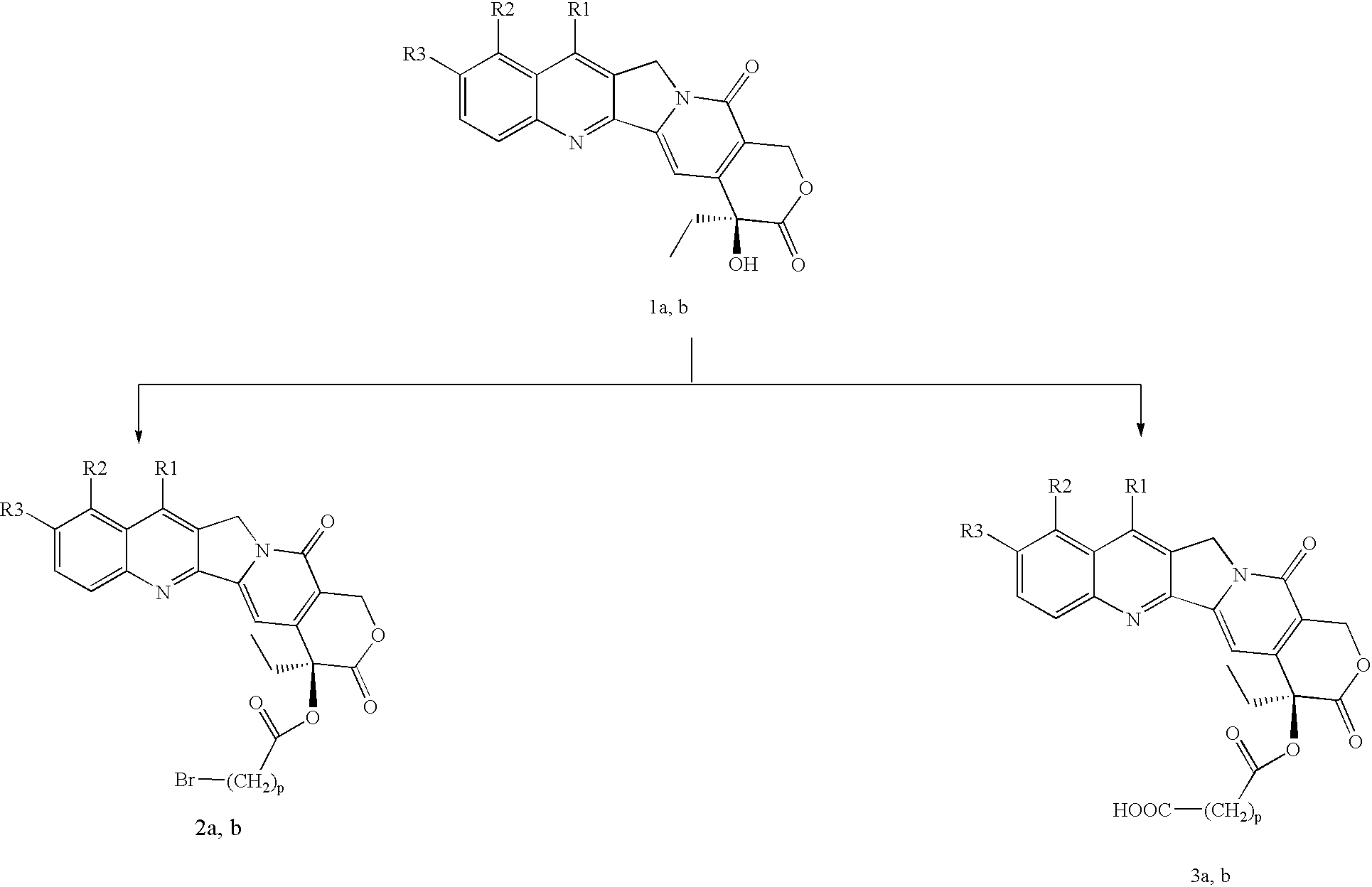
Peptide conjugates of cytotoxins as therapeutics
ActiveUS20210009719A1Organic active ingredientsPeptide/protein ingredientsDiseaseTopoisomerase-I Inhibitor
The present invention relates to peptide conjugates of cytotoxins such as topoisomerase I inhibitors which are useful for the treatment of diseases such as cancer.
Owner:CYBREXA 2 INC
Azaindenoisoquinoline topoisomerase I inhibitors
ActiveUS9034870B2Increase distanceProlonged covalent attachmentBiocideOrganic chemistryDNA underwindingIsoquinoline
The invention described herein pertains to substituted azaindenoisoquinoline compounds, in particular 7-, 8-, 9-, and 10-azaindenoisoquinoline compounds, which are inhibitors of topoisomerase I, processes and intermediates for their syntheses, pharmaceutical compositions of the compounds, and methods of using them in the treatment of cancer.
Owner:NAT INST OF HEALTH REPRESENTED BY THE SEC OF THE DEPT OF HEALTH & HUMAN SERVICES NAT INST OF HEALTH +1
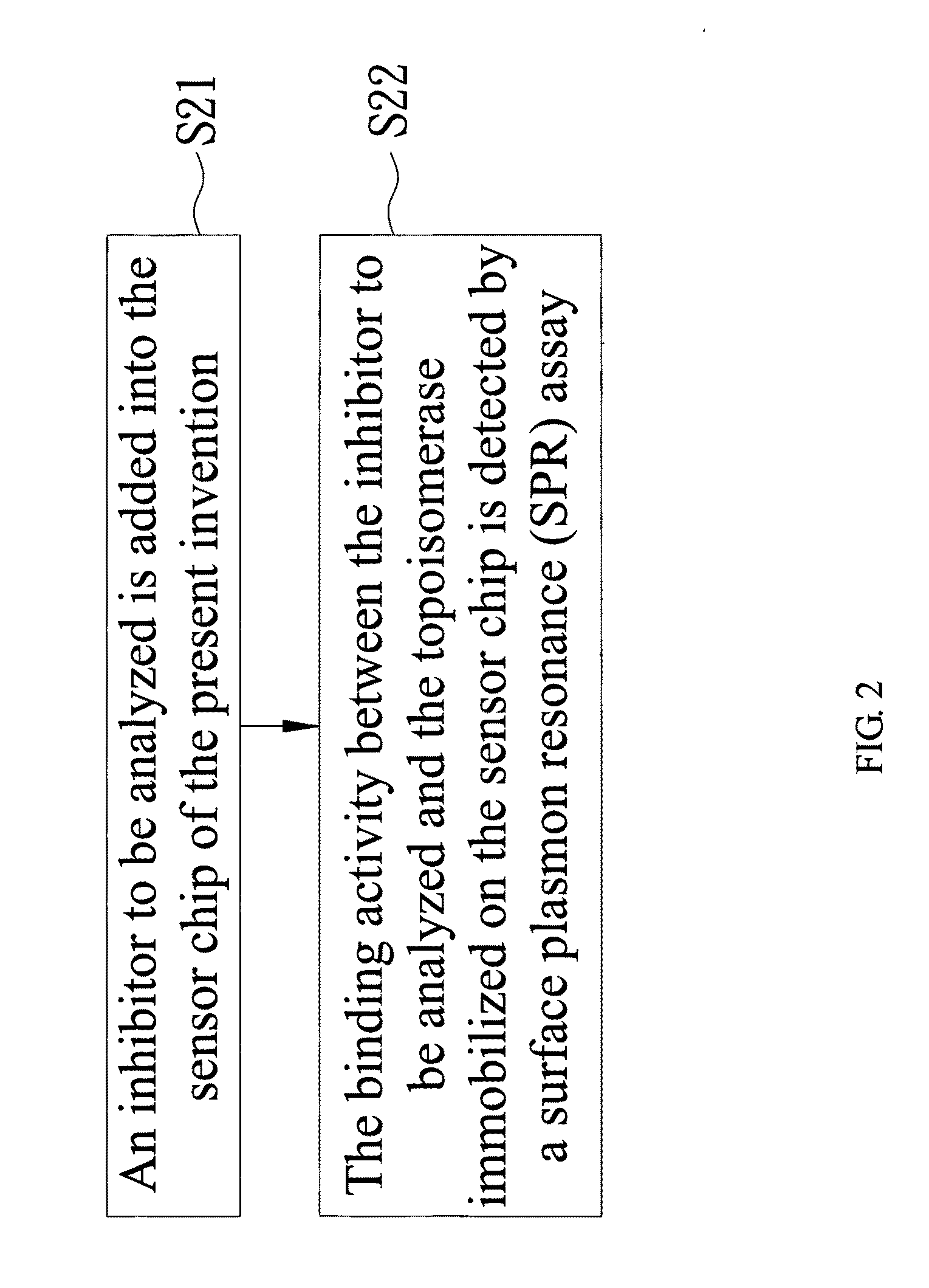
Sensor chip for screening topoisomerase inhibitor and screening method thereof
ActiveUS20110287438A1Rapid and convenient efficiencyReduce difficultyCompound screeningApoptosis detectionDNA underwindingTopoisomerase-I Inhibitor
The present invention discloses a sensor chip for screening a topoisomerase inhibitor and a screening method thereof. The sensor chip comprises topoisomerase I and a biochip. The topoisomerase I is immobilized on the biochip, and the topoisomerase I has DNA catalytic activity. This provides a rapid screening method for topoisomerase I inhibitors.
Owner:TAIPEI MEDICAL UNIV
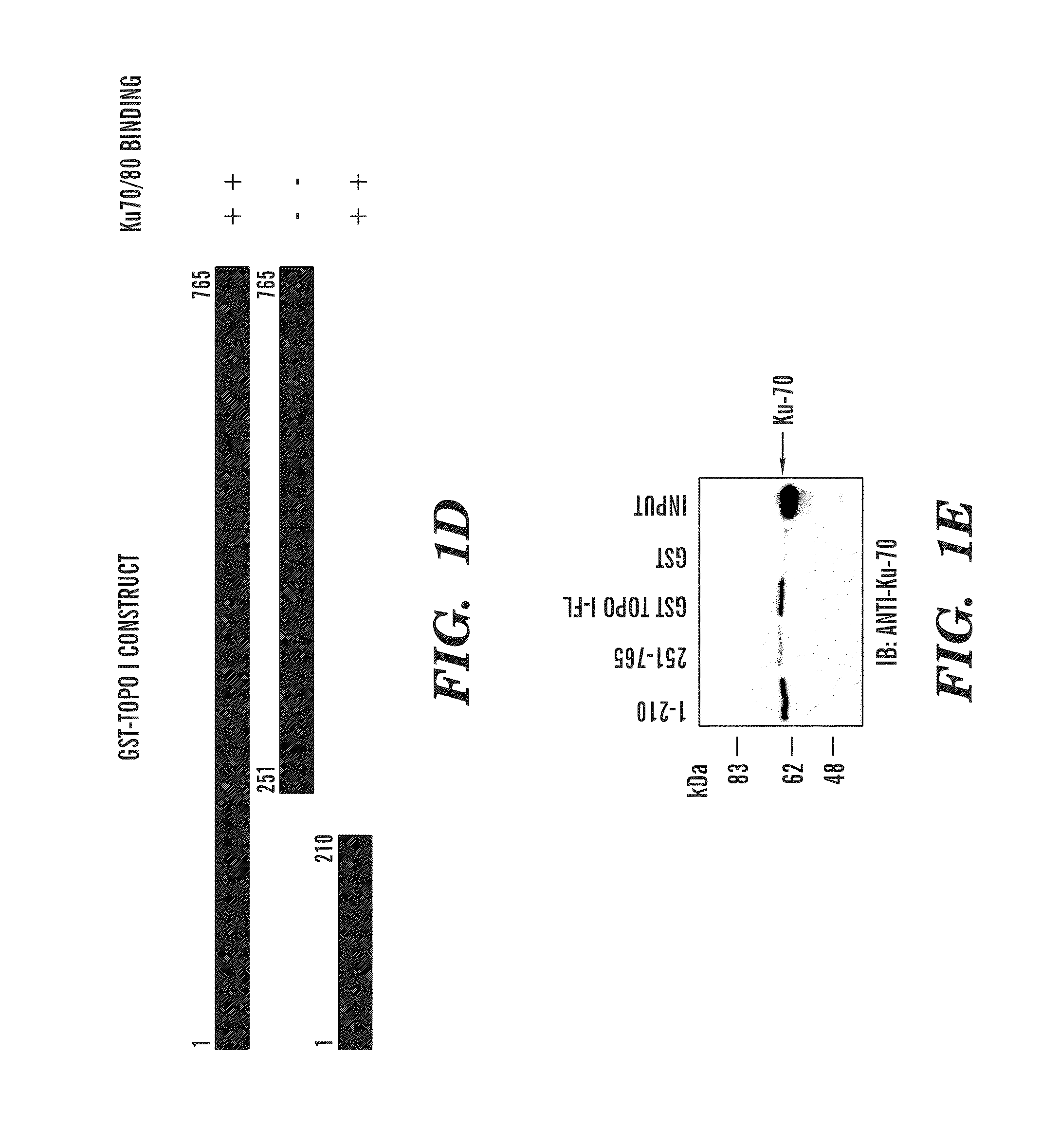
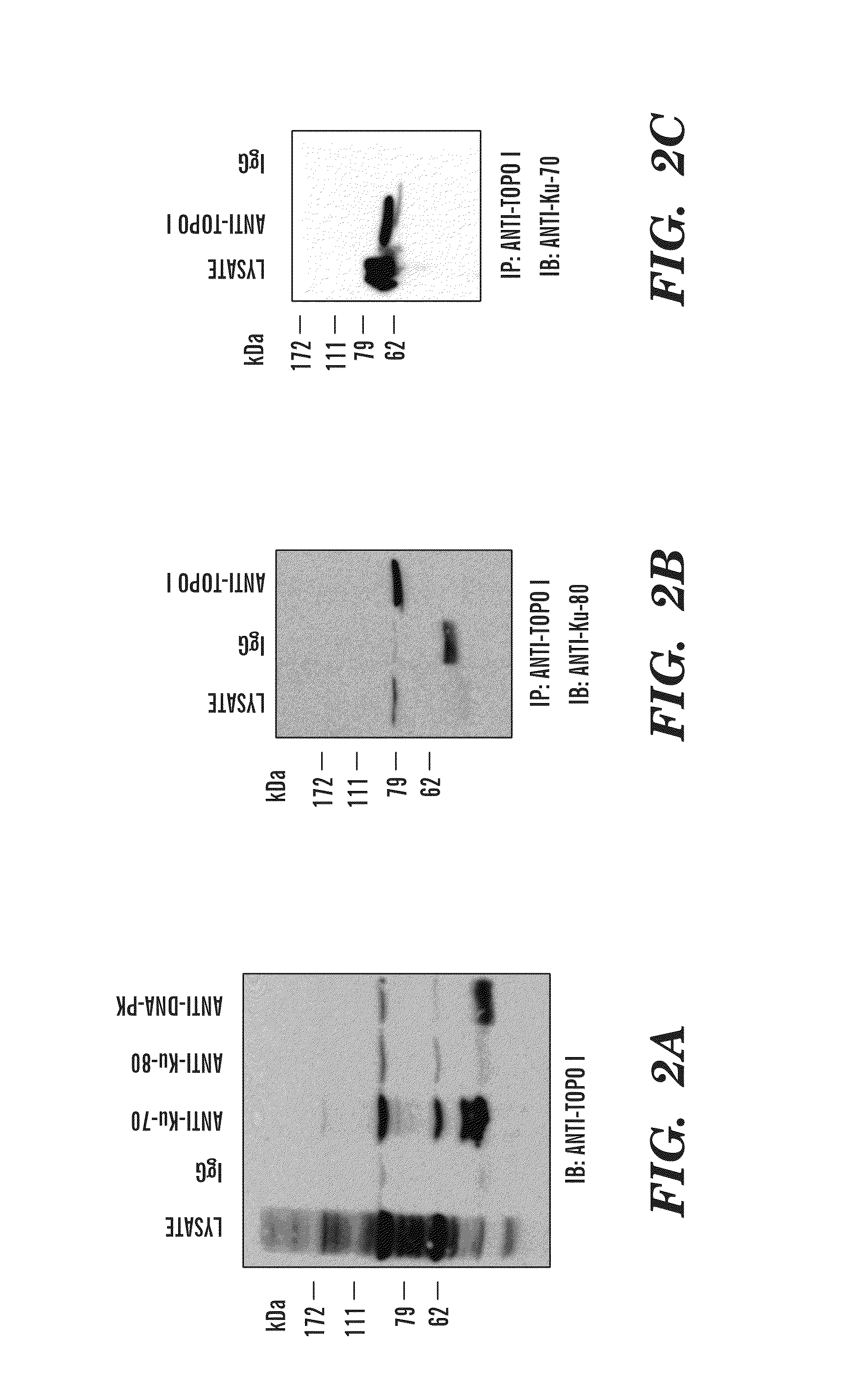
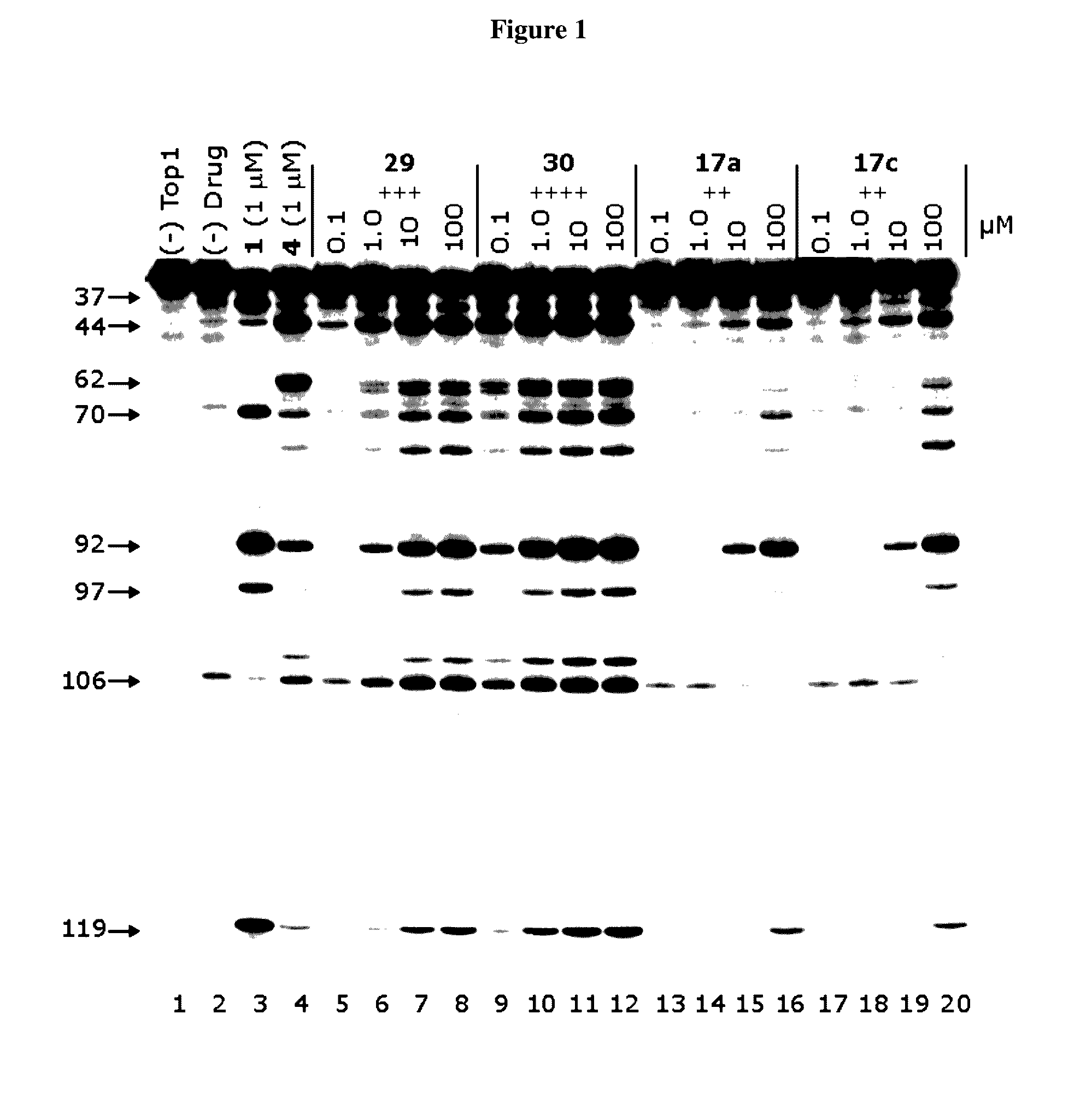
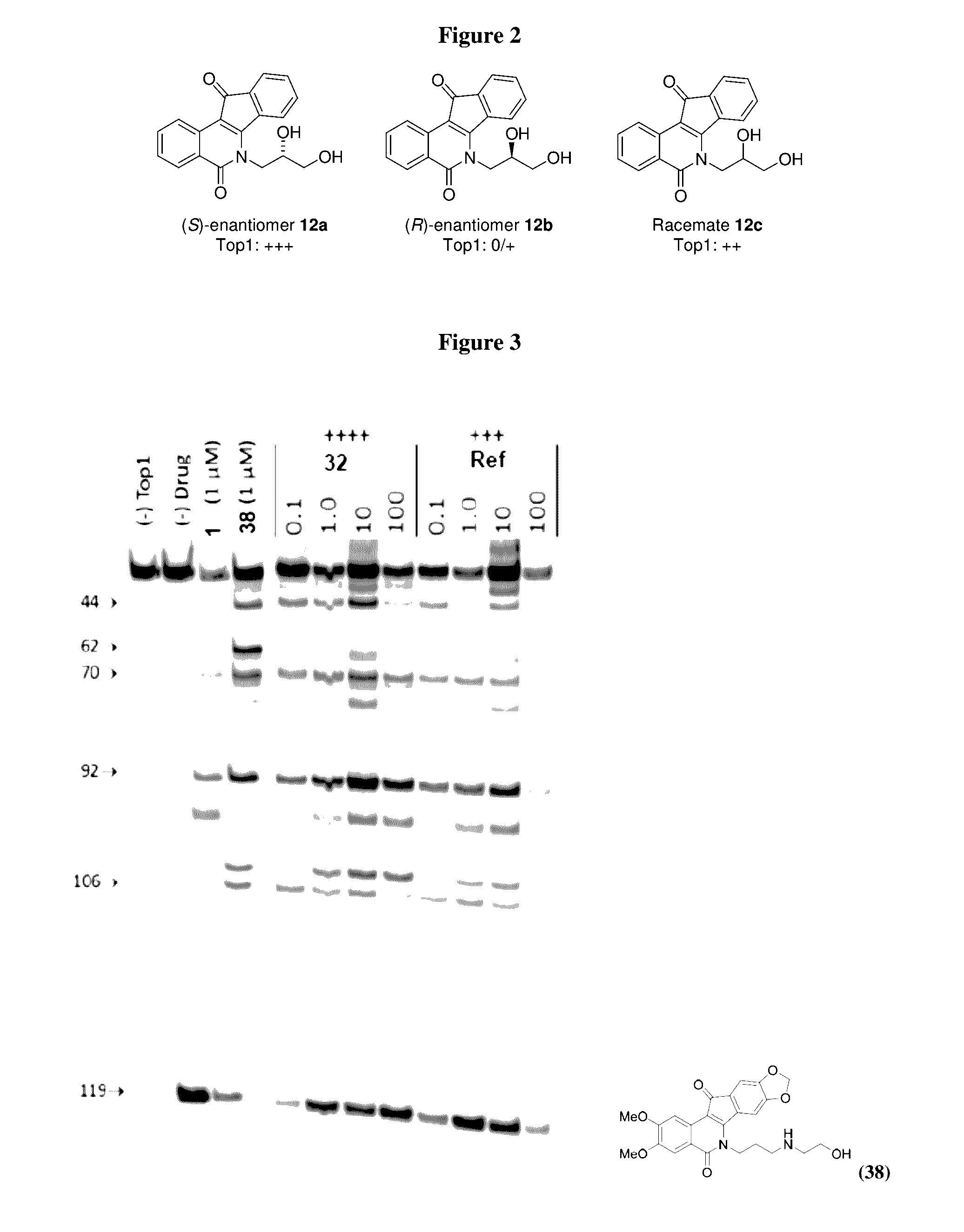
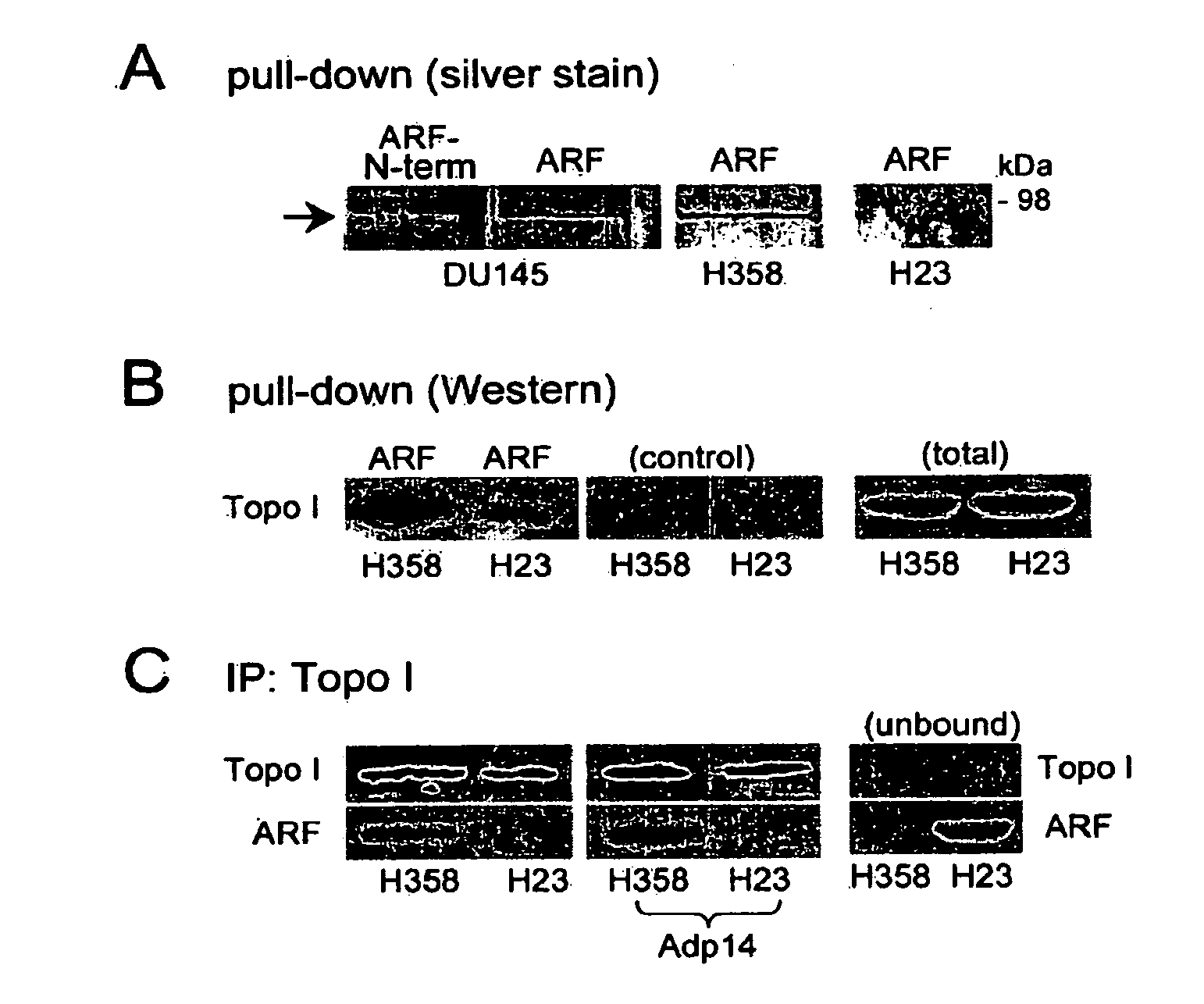
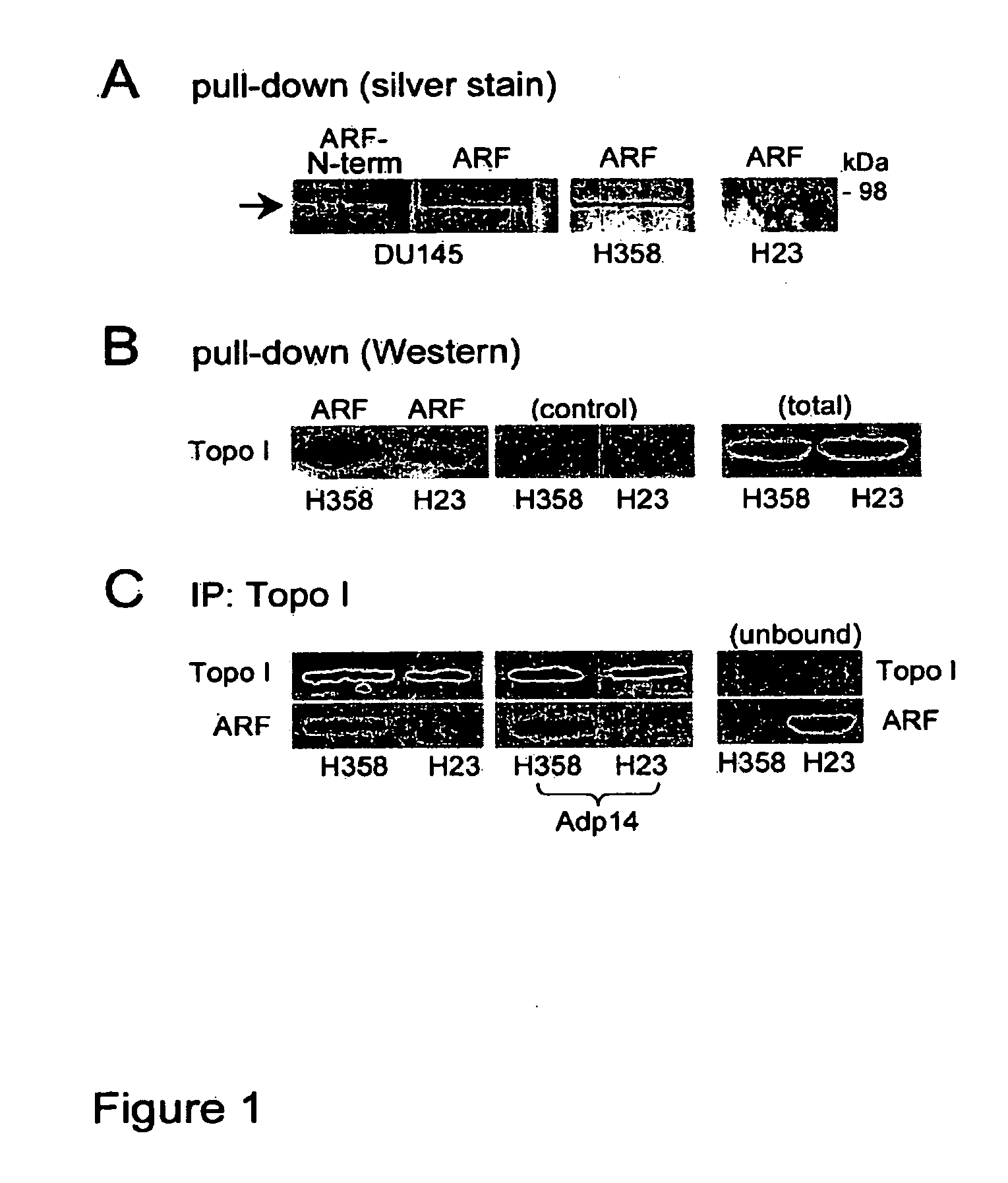
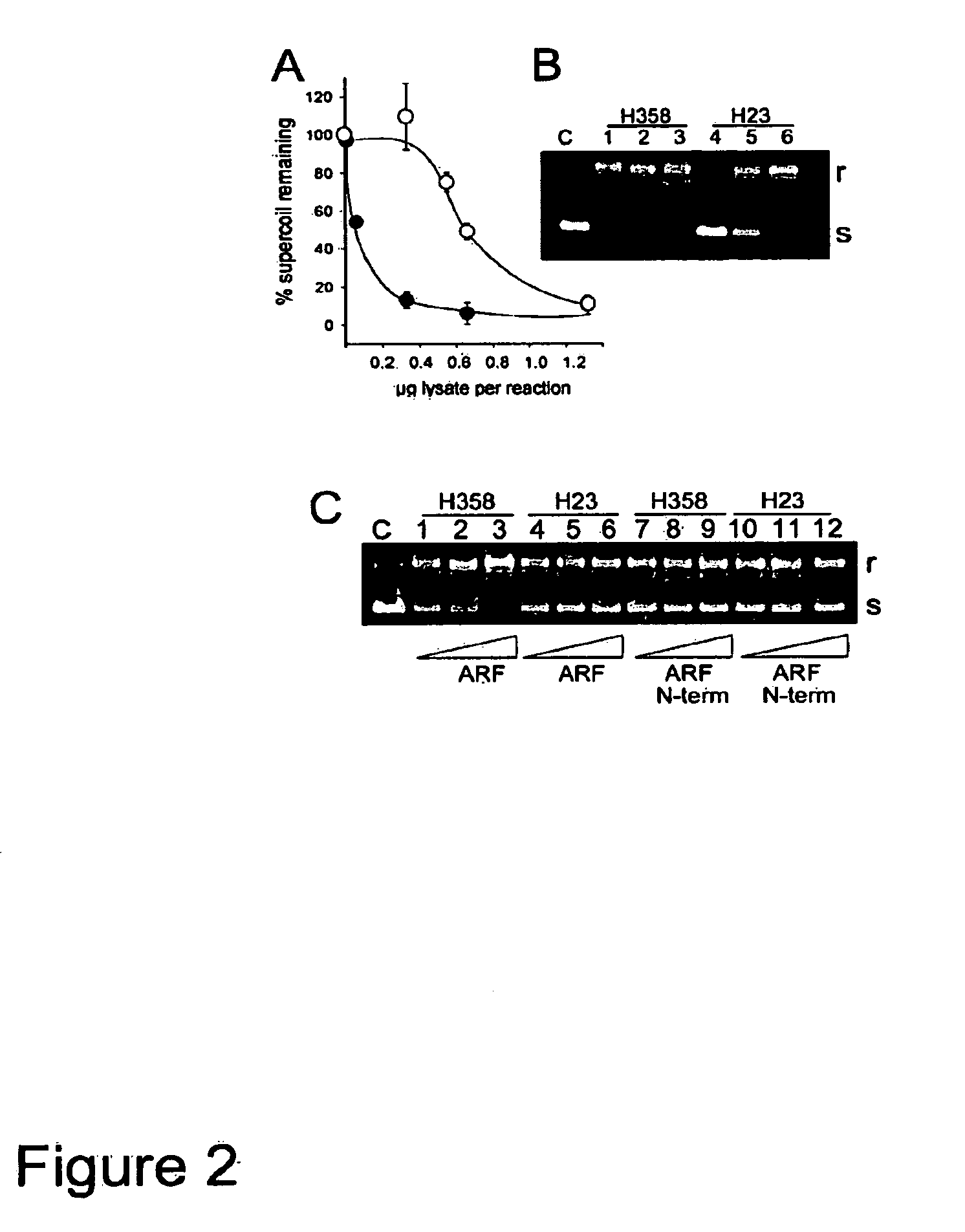
Methods and compositions for topoisomerase i modulated tumor suppression
InactiveUS20110028536A1Less sensitive (or insensitive)Reduce capacityBiocidePeptide/protein ingredientsDNA underwindingTopoisomerase-I Inhibitor
Disclosed herein are methods and compositions for enhancing the sensitivity of cells to the effects of topoisomerase I inhibitors. Also disclosed are methods and compositions for inducing apoptosis and / or growth arrest which may be used for tumor suppression.
Owner:RG BIOPHARMA
Cancer therapy using a combination of HSP90 inhibitors with topoisomerase I inhibitors
ActiveUS9439899B2Increasing side effect profileSurprising biological activityHydroxy compound active ingredientsAntineoplastic agentsDNA underwindingTopoisomerase-I Inhibitor
Owner:SYNTA PHARMA CORP
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com