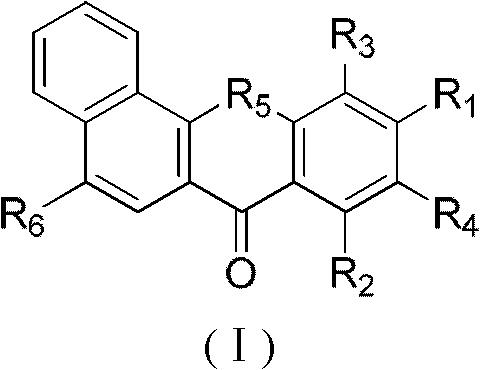
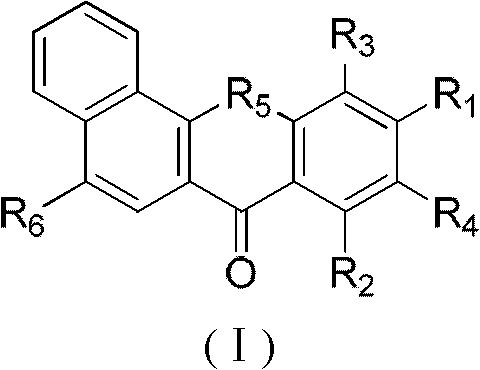
Substituted benzoxanthone type compound and application thereof
A kind of technology of benzoxanthone and compound, applied in benzoxanthone compound and its application field in pharmacy, can solve the problem such as in vivo activity reduction, toxicity, achieve the effect of good antitumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
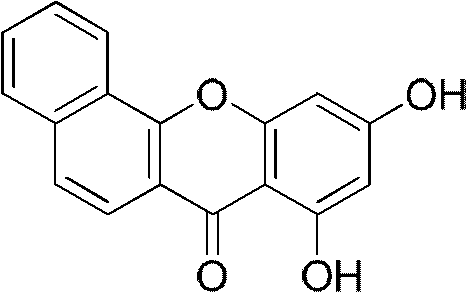
[0067] Example 1: Synthesis of 8,10-hydroxybenzoxanthone
[0068] Put 10g of 1-hydroxy-2-naphthoic acid, 10g of phloroglucinol and 30g of zinc chloride in 70mL of phosphorus oxychloride, react at 70°C for 6 hours, stop the reaction, add 1.5L of water after cooling, stir overnight, pump A dark red solid was obtained by filtration and purified by column chromatography (THF:PE 1:8) to obtain 5.3 g of light yellow solid 8,10-hydroxybenzoxanthone (35.8%). 1 H NMR (500MHz, CDCl 3 ), δ12.91(s, 1H), 10.24(s, 1H), 8.62(d, J=7.9Hz, 1H), 8.14(d, J=8.7Hz, 1H), 7.94(d, J=7.9Hz , 1H), 7.72 (m, 3H), 6.60 (d, J=1.9Hz, 1H), 6.36 (d, J=1.9Hz, 1H).
Embodiment 2
[0069] Example 2: Synthesis of 8-hydroxy-10-methoxy-7H-benzoxanthone
[0070] According to the method of Example 1, 3,5-dimethoxyphenol was used instead of phloroglucinol to obtain 5.6 g of light yellow solid 8-hydroxyl-10-methoxyl-7H-benzoxanthone (36.1%) .
[0071] 1 H NMR (500MHz CDCl 3 ), δ12.94(s, 1H), 8.64(d, J=7.9Hz, 1H), 8.19(d, J=8.7Hz, 1H), 7.95(d, J=7.9Hz, 1H), 7.72(m , 3H), 6.65(d, J=1.9Hz, 1H), 6.43(d, J=1.9Hz, 1H), 3.94(s, 1H).
Embodiment 3
[0072] Example 3: Synthesis of 9,11-dibromo-8,10-dihydroxy-7H-benzoxanthone
[0073] Add 0.50g of 8,10-hydroxybenzoxanthone to 10mL of acetic acid, add 0.18mL of bromine dropwise, react at room temperature for 2h, and filter with suction to obtain 0.71g of yellow solid 9,11-dibromo-8,10-dihydroxy -7H-Benzoxanthone (91.0%). 1 H NMR (500MHz, CDCl 3 ) δ 7.70 (m, 3H), 7.91 (m, 1H), 8.16 (d, J=8.7Hz, 1H), 8.74 (m, 1H), 13.97 (s, 1H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com