Novel topoisomerase I inhibitor and pharmaceutical compositions thereof as well as preparation methods and applications of novel topoisomerase I inhibitor and pharmaceutical compositions thereof
A technology of topozyme and drug, applied in the direction of drug combination, organic chemical method, separation/purification of carbonyl compound, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
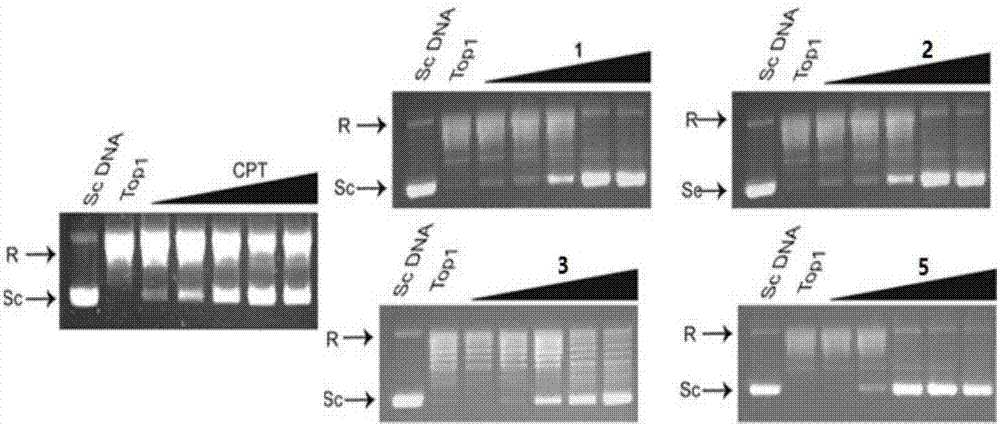
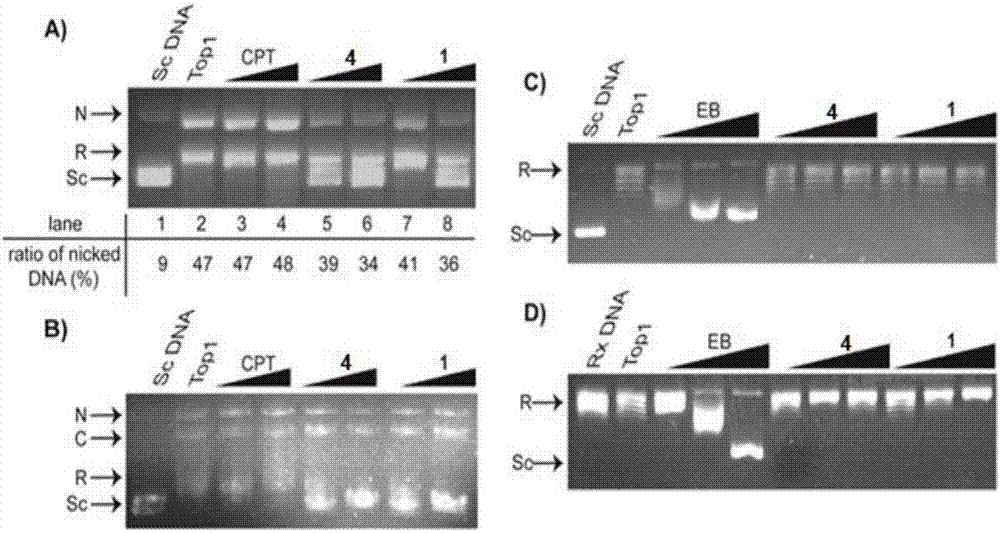
Method used
Image
Examples
Embodiment 1
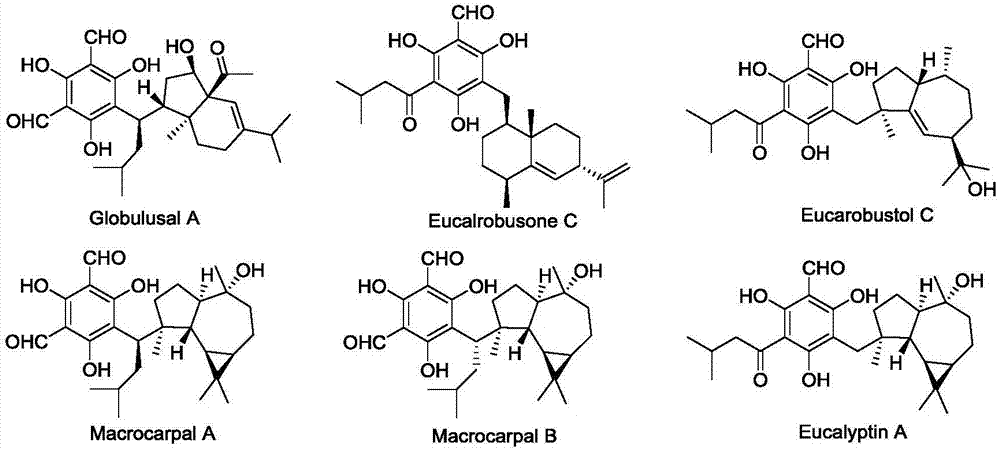
[0031] Preparation of three compounds of Eucalyptus globulus heteroterpene A (Globulusal A), Macrocarpal A and Macrocarpal B:
[0032]Take blue eucalyptus fruit (6.0kg), crush it, and extract it by cold soaking in ethyl acetate for 3 times, each time for 48 hours, combine the extracts, recover the solvent under reduced pressure to obtain extract (550g). The extract was mixed with silica gel 80-100 mesh, followed by silica gel (200-300 mesh) column chromatography, the eluent was petroleum ether-acetone (30:1→1:1, v / v) gradient elution, TLC Detection combined to obtain nine components Fr.1-Fr.9. Fr.9 (40g) was subjected to Rp-18 reverse phase column chromatography (MeCN–H 2 O, 50:50 → 100:0 v / v) yielded 6 fractions (Fr.9a–Fr.9f). Fr.9d (1.8g) was chromatographed by Sephadex LH-20 (CHCl 3 –MeOH, 1:1v / v) followed by reverse phase semi-preparative HPLC (MeCN–H 2 O, containing 0.01% TFA, 80:20→95:5v / v) was further purified to obtain eucalyptus heteroterpene A (1, 9.0mg). Compou...
Embodiment 2
[0034] Preparation of a compound of Eucalyptin A:
[0035] Take blue eucalyptus fruit (6.0kg), crush it, and extract it by cold soaking in ethyl acetate for 3 times, each time for 48 hours, combine the extracts, recover the solvent under reduced pressure to obtain extract (550g). The extract was mixed with silica gel 80-100 mesh, followed by silica gel (200-300 mesh) column chromatography, the eluent was petroleum ether-acetone (30:1→1:1, v / v) gradient elution, TLC Detection combined to obtain nine components Fr.1-Fr.9. Fr.5 (20g) by Sephadex LH-20 (CHCl 3 -MeOH, 3:2v / v) after removing fatty acid, it was subjected to Rp-18 reverse phase column chromatography (MeCN–H 2 O, 60:40 → 100:0 v / v) yielded 6 fractions (Fr.5a–Fr.5f). Fr.5e (50mg) was reversed phase semi-preparative by HPLC (MeCN–H 2 O, H 2 O containing 0.01% TFA, 75:25→95:5 v / v) was further purified to obtain Eucalyptin A (6, 13 mg).
Embodiment 3
[0037] Preparation of two compounds Eucalrobusone C and Eucarobustol C:
[0038] Take blue eucalyptus fruit (6.0kg), crush it, and extract it by cold soaking in ethyl acetate for 3 times, each time for 48 hours, combine the extracts, recover the solvent under reduced pressure to obtain extract (550g). The extract was mixed with silica gel 80-100 mesh, followed by silica gel (200-300 mesh) column chromatography, the eluent was petroleum ether-acetone (30:1→1:1, v / v) gradient elution, TLC Detection combined to obtain nine components Fr.1-Fr.9. Fr.4 (30g) by Sephadex LH-20 (CHCl 3 -MeOH, 3:2v / v) after removing fatty acid, it was subjected to Rp-18 reverse phase column chromatography (MeCN–H 2 O, 60:40 → 100:0 v / v) yielded 5 fractions (Fr.4a–Fr.4e). Fr.4d (128mg) was reversed phase semi-preparative by HPLC (MeCN–H 2 O, H 2 O containing 0.01% TFA, 90:10→99:1 v / v) was further purified to give Eucalrobusone C (2, 8.2 mg) and Eucarobustol C (3, 6.5 mg).
[0039]
[0040] Phys...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com