Antitumor antibiotic ancomycin and its derivative
A technology of ancomycin and its derivatives, applied in the field of antitumor antibiotic ancomycin and its derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1 > Preparation of Ancomycin
[0047] Streptomyces strain C-9095 preserved in the glycerol tube was inoculated to the slant (glucose 1%, asparagine 0.1%, dipotassium hydrogen phosphate 0.05%, agar 1.5%, pH 7.2-7.4, sterilized, and cultivated at 28°C for 7-10 day) culture medium, cultivated in a constant temperature incubator at 28°C for 7-10 days. Inoculate the slant culture into 100ml primary seed medium (glucose 10g, soluble starch 15g, beef extract 5g, fish peptone 5g, soybean cake powder 10g, sodium chloride 3g, adjust pH to 7.0, double distilled water into 1000ml, autoclaved), 28 ℃ at 28 ℃ in the rotary shaker of 180 rev / min and cultivated for 48 hours, got the first-grade seeds that cultivated, and replanted in 100ml secondary seed culture medium by 10% inoculum size ( Seed culture medium of the same level), cultured on a rotary shaker at 28°C for 24 hours. Get the secondary seeds that have been cultivated, and replant in 1000ml fermentation medium by 1...
Embodiment 2
[0049] Example 2 > Preparation of Ancomycin Methyl Ester
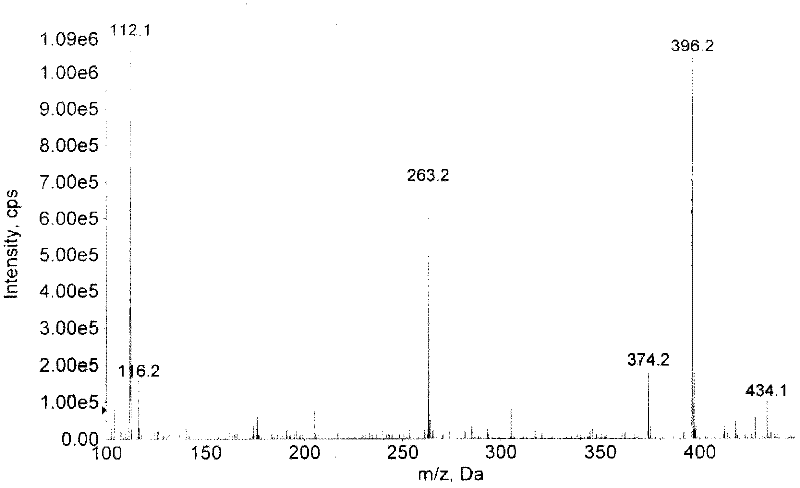
[0050] Take 12ml of methanol in the reaction flask, slowly add thionyl chloride (SOCl 2 ) 3ml, the temperature was controlled below 0°C, and the reaction was continued for 1 hour at about 0°C after the dropwise addition was completed. Weigh 500 mg of ancomycin, dissolve in methanol-thionyl chloride solution, stir at room temperature, and track the reaction by TLC, solvent methanol: ethyl acetate: ammonia water (5:3:2), total reaction 48h, reduce Evaporate under pressure to obtain a yellow oily liquid. Dissolve 2ml of the yellow oily solution in methanol, then add 20ml of diethyl ether, a white solid is precipitated, and the solid is obtained by suction filtration under reduced pressure. The solid matter is separated with a preparative thin-layer silica gel chromatography plate, and the developing solvent is methanol: ammonia water=5: 1, and R f =0.50 band, eluting with the eluent of ethanol: water=3: 2, and the elu...
Embodiment 3
[0051] Example 3 > Preparation of Ancomycin Isopropyl Ester
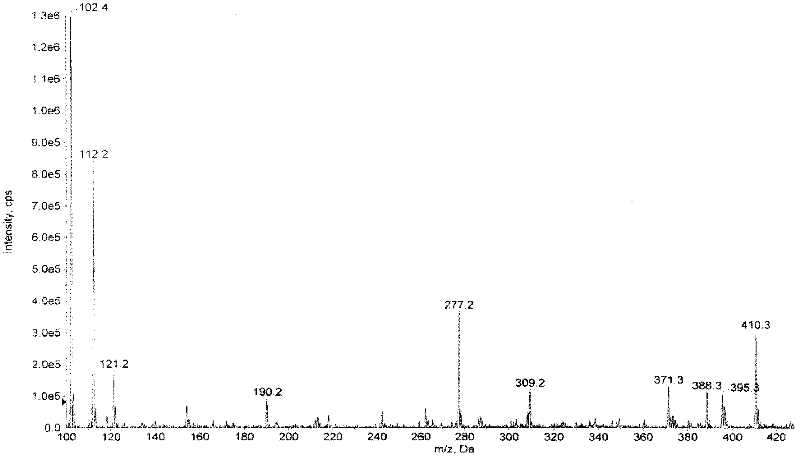
[0052] Put 200 mg of ancomycin and 2 ml of dimethyl sulfoxide (DMSO) into the reaction bottle, stir until dissolved in a warm state, and slowly add 3 ml of aceton (aceton) dropwise without obvious turbidity. Then add 1ml iodoisopropane and 100mg K 2 CO 3 Powder, the reaction solution is slightly turbid, keep the internal temperature (50-55) ° C, stir and react overnight, and react for a total of 17 hours. The reaction solution was evaporated to dryness under reduced pressure to obtain a solid, which was separated by preparative thin-layer silica gel chromatography. The developing solvent was methanol: ethyl acetate: ammonia water = 2: 0.4: 0.1, and R f =0.35 band, eluted with methanol, and the eluate was evaporated to dryness under reduced pressure to obtain 28 mg of ancomycin isopropyl ester. ESI-MS m / z416[M+H] + (molecular formula C 16 h 25 N 5 o 8 , molecular weight is 415).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com