Antitumor antibiotics, pharmaceutically acceptable salts thereof, preparation method thereof and use thereof
A glycopeptide antibiotic and pharmaceutical technology, which is applied in the direction of antineoplastic drugs, microbial-based methods, biochemical equipment and methods, etc., and can solve problems such as application limitations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
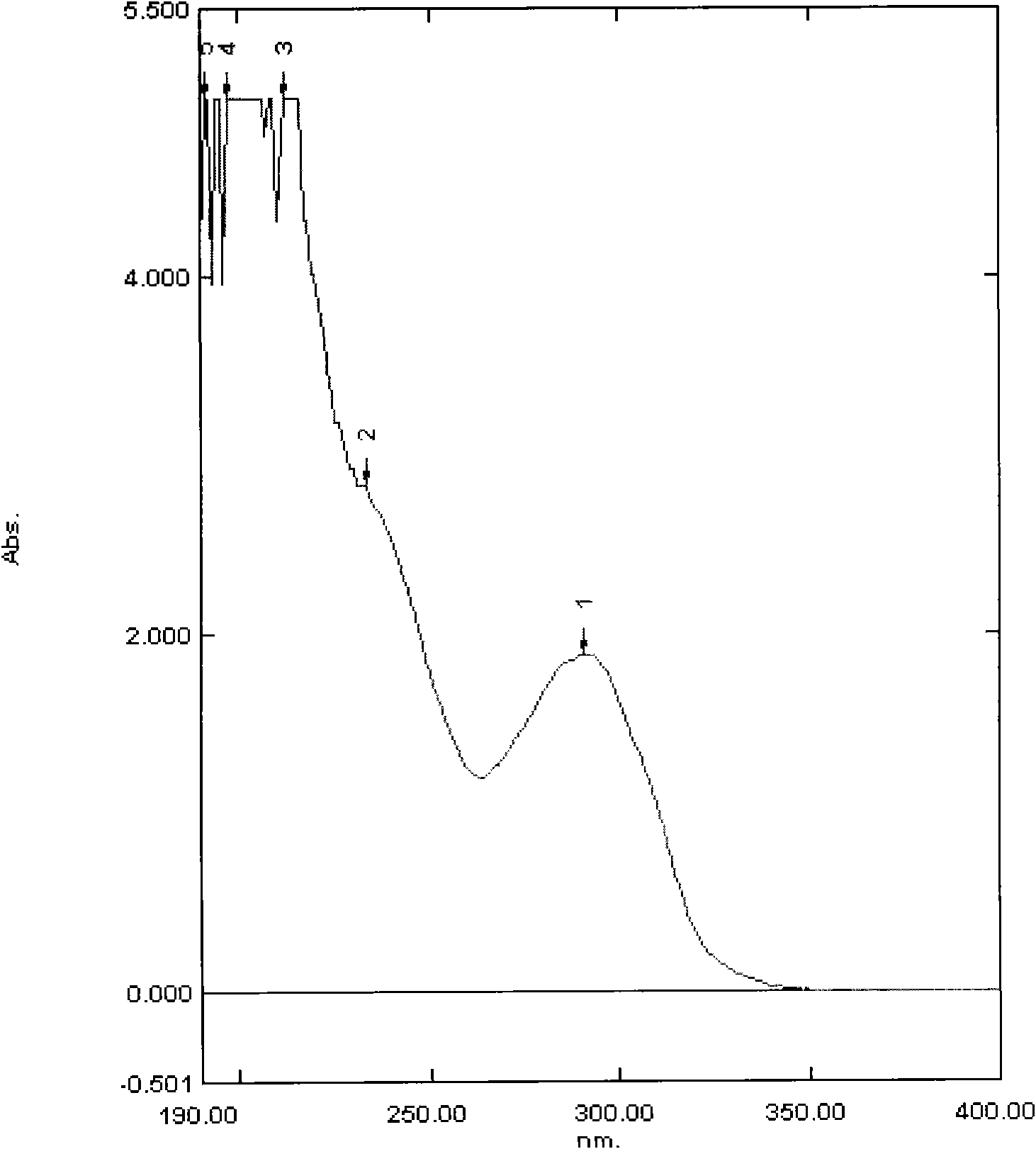
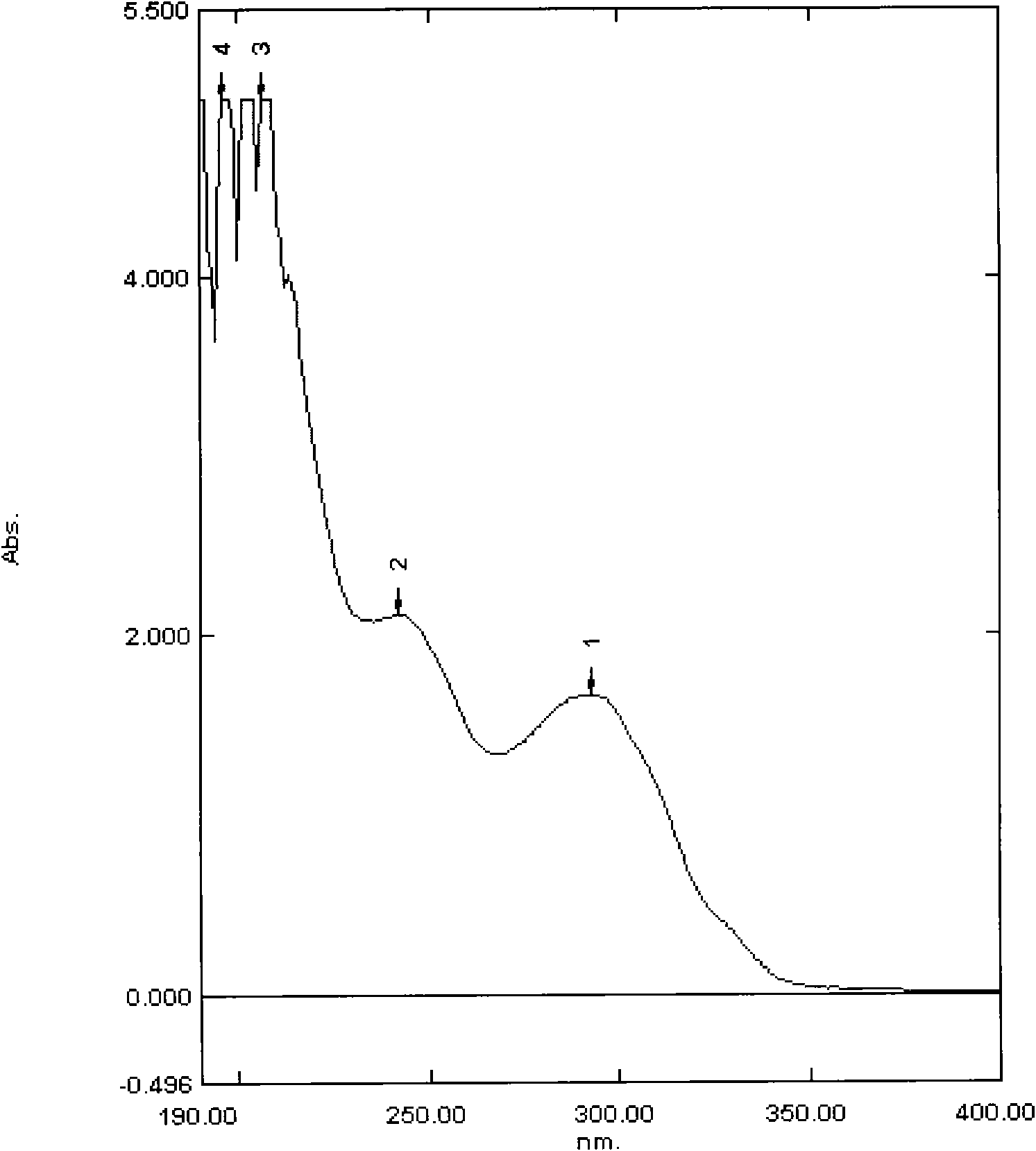
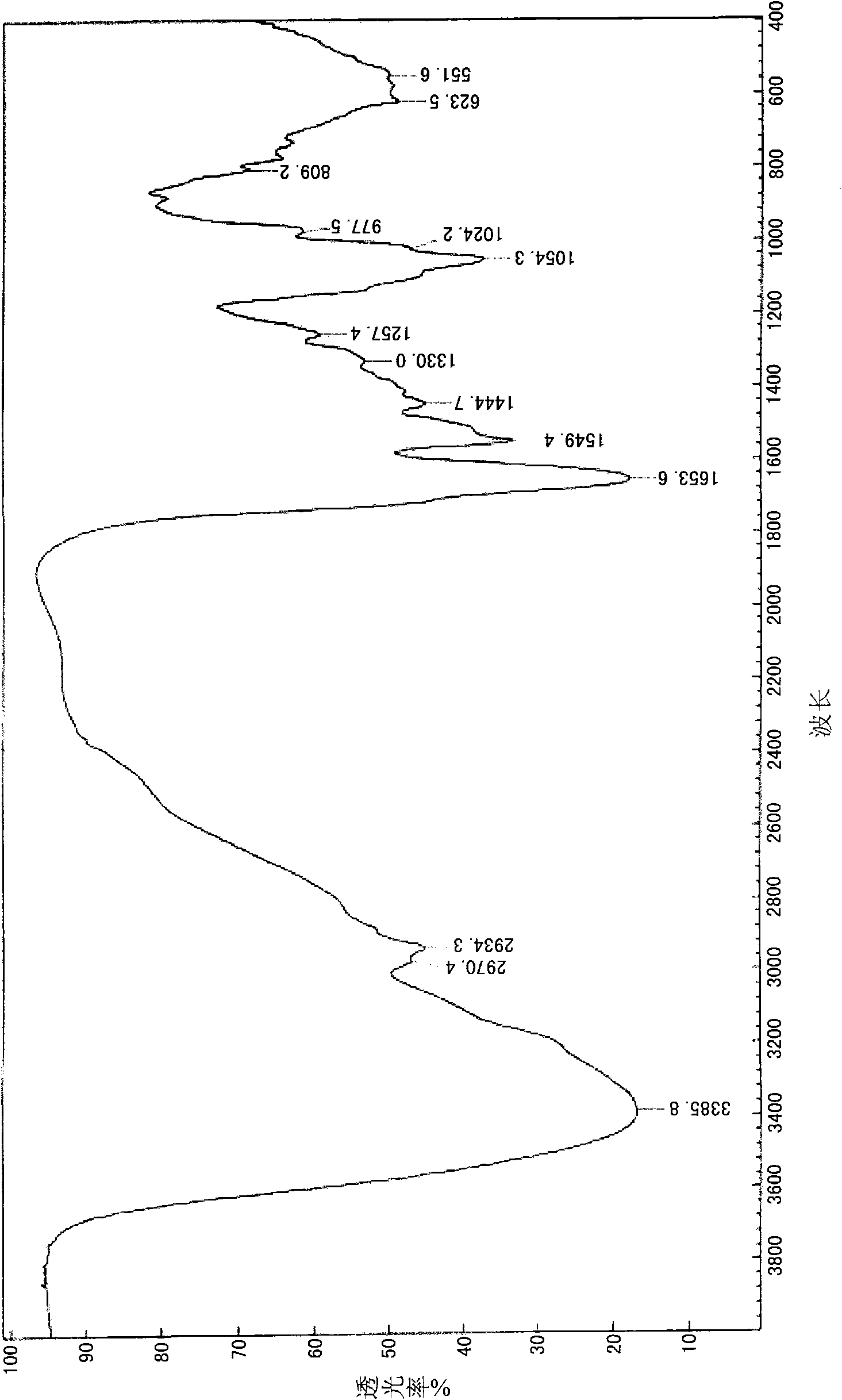
Image
Examples
Embodiment 1
[0074] Example 1 Fermentation
[0075] Glucose 1.0%; peptone 0.5%; starch 1.0%; NaCl 0.5%; agar 2.0% (pH7.2-7.5) as slant medium, sterilized at 120°C for 30 minutes to make slant, and kept at 37°C for 3 days . When the surface moisture is slightly dry and there is no growth of miscellaneous bacteria, inoculate Streptomyces verticillus var. Pingyangensis n.sp with spores of Streptomyces verticillus var. Pingyangensis n.sp, and cultivate at 28°C for 8 days. It is fluffy and can be collected and used when there is no bacteria. Fill 100ml medium respectively in a plurality of 500ml glass bottles (medium composition: starch 2.5%, glucose 0.5%, bean cake powder 3.5%, KH 2 PO 4 0.1%, ZnSO 4 0.05%, CuSO 4 0.01%, pH natural). Add a cotton plug, sterilize at 120°C for 30 minutes, and inoculate by digging blocks from an inclined plane. After 48 hours of rotary culture in a rotary shaker at 28°C, they were used as seeds.
[0076] Then with the fermenting medium (pH nature) of th...
Embodiment 2
[0077] Example 2 Extraction and Purification
[0078] The fermented culture liquid (25 liters) of embodiment 1 is adjusted pH2~3 with oxalic acid solution, filters, and filtrate passes through 122 (H + ) cation exchange resin (1 liter) adsorption, and then eluted with 0.3mol / L HCl (4 liters), the active part was adjusted to pH 7.0, and an appropriate amount of CuSO was added 4 solution to fully chelate Cu 2+ , and then desalted with macroporous adsorption resin, eluted with 20% acetone solution containing 0.01M HCl, collected the eluate and removed acetone and carried out CM-SephadexC-25 (NH 4 + ) column chromatography, with 0.1 ~ 1M NH 4 Cl gradient elution, separated to obtain NC0604 copper-containing eluate, and then desalted by macroporous adsorption resin, the desalted eluate was vacuum concentrated and freeze-dried to obtain blue NC0604 copper-containing pure product (313mg). The copper-containing product is decopper-treated with diphenylsulfocarbezone in acidic meth...
Embodiment 3
[0079] Embodiment 3 extraction and purification
[0080] The fermented culture liquid (25 liters) of embodiment 1 is adjusted pH2~3 with oxalic acid solution, filters, and filtrate passes through 151 (H + ) ion exchange resin (1 liter) adsorption, washed with 0.3mol / L HCl (4 liters) to elute, the active part adjusts the pH to 6.5 and adds an appropriate amount of CuSO 4 solution to fully chelate Cu 2+ , then desalted with macroporous adsorption resin 4006, eluted with 10% acetone solution containing 0.01M HCl, collected the eluate, adjusted pH 6.5 with ammonia water after removing acetone, and carried out CM-Sephadex C-25 (NH 4 + ) column chromatography, after washing the column with water, use 0.1 ~ 1M NH 4 Cl gradient elution, collected according to the peak position, combined. The NC0604 copper-containing eluate was separated, and then desalted by macroporous adsorption resin. The desalted eluate was vacuum-concentrated and freeze-dried to obtain the blue NC0604 copper-...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com