Antharcycline antitumor antibiotics loaded nano-micelle preparation and preparation method thereof
A nanomicelle, anti-tumor technology, applied in the field of biomedical materials, can solve the problems of low drug loading, dissociation, etc., achieve uniform particle size, improve stability, and increase loading capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
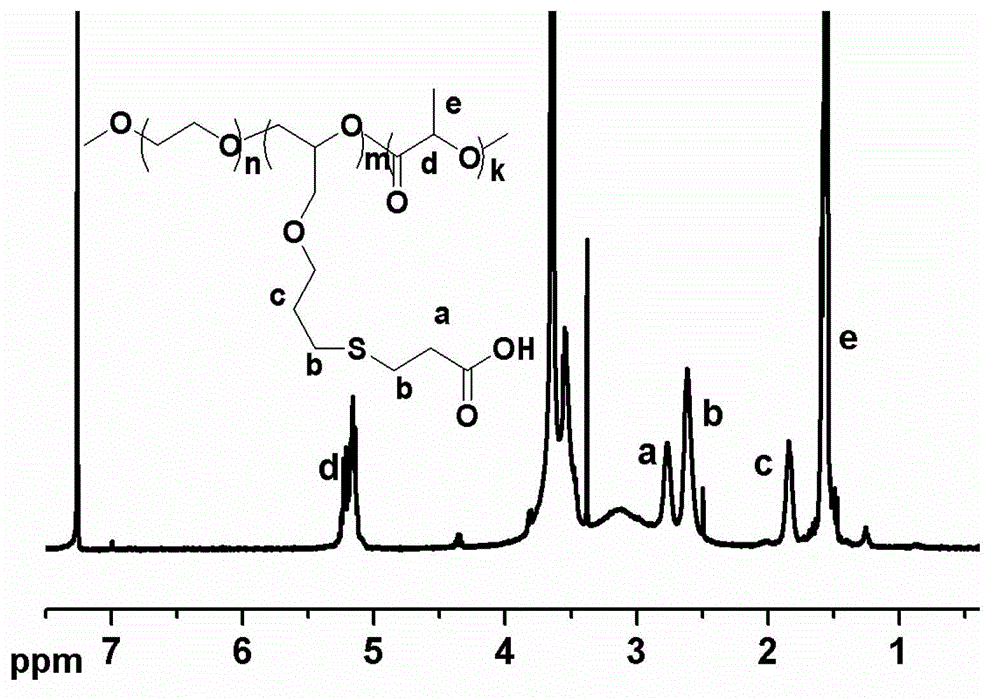
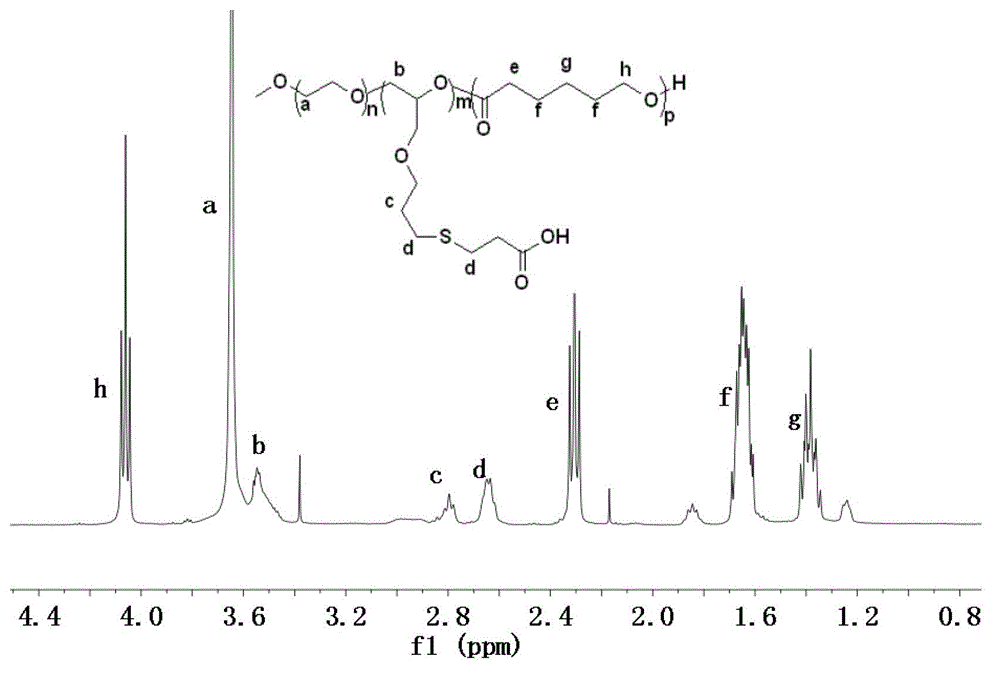
[0034] The preparation method of the A-B-C type triblock polymer of the present invention is a known technology, and the specific preparation steps are as follows:
[0035] Step 1: Under the protection of nitrogen, put PEGs of different molecular weights into the reaction vessel, add the solvent toluene for azeotropic water removal, after 2-5 hours, add cerium hydroxide into the reaction vessel, and stir the reaction at 40°C-70°C After 2-4 hours, remove the solvent, continue to add allyl glycidyl ether, react at 20°C-50°C for 12-24 hours, cool to room temperature, settle with 200-500mL ether, filter, and vacuum dry to obtain solid powder PEG-PAGE , the molar ratio of PEG, cerium hydroxide and allyl glycidyl ether is 1:1:4-20;
[0036] The second step: Take the above PEG-PAGE and dissolve it in 10-50 mL of THF. After stirring and dissolving, add the catalyst Zn[N(SiMe 3 ) 2 ] 2 After reacting with polyester monomer and stirring at room temperature for 2-10 hours, settle with...
Embodiment 1
[0051] Example 1: Preparation of nanomicelles encapsulating doxorubicin with A-B-C type triblock polymers of different molecular weights
[0052] Table 1
[0053]
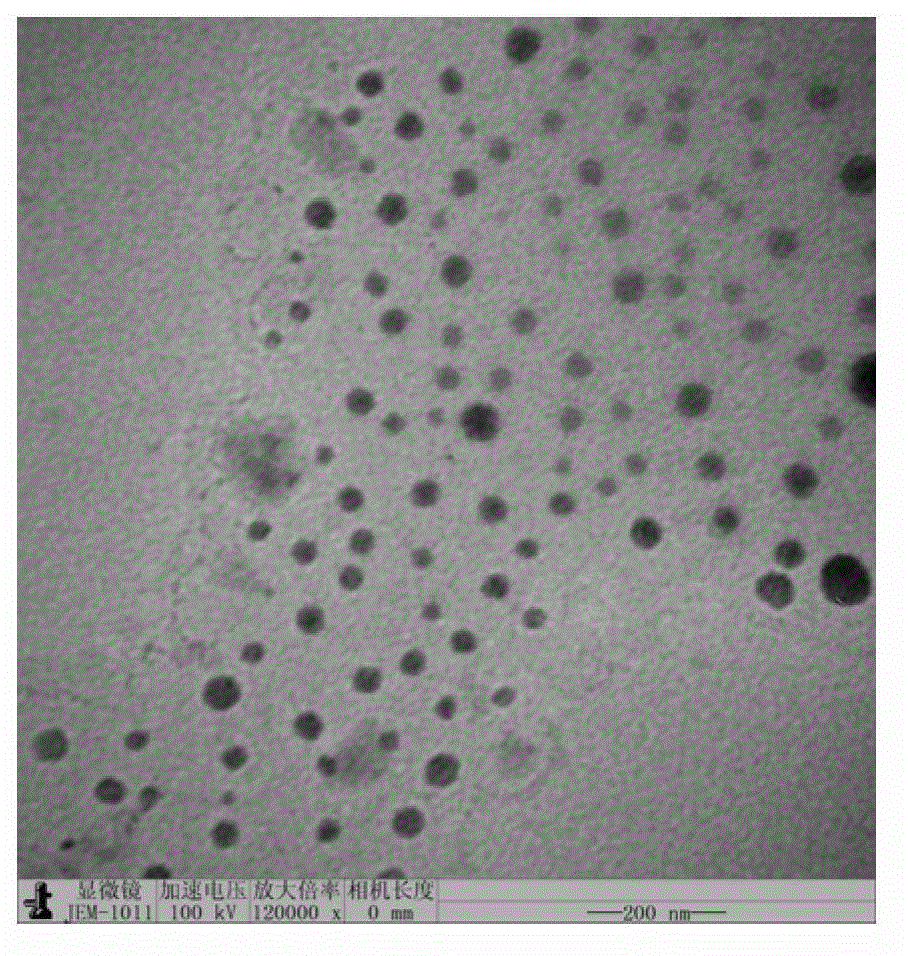
[0054] According to the formula in Table 1, 100 mg of A-B-C triblock polymer was weighed and dissolved in acetonitrile, and placed in a round-bottomed flask. The concentration of the A-B-C triblock polymer was 1 mg / mL. , evaporated the organic solvent to form a thin and uniform polymer film on the surface of the round bottom flask, dissolved 10 mg doxorubicin (ADM) in water to obtain an aqueous solution of doxorubicin, the concentration of ADM was 0.1 mg / mL, Add the aqueous solution of doxorubicin to the round bottom flask, 60 o Hydrate at C for 1 min, filter and sterilize with a 0.22 um microporous membrane to obtain a nanomicelle solution, and freeze-dry to obtain a nanomicelle lyophilized powder loaded with anthracycline antitumor antibiotics. The obtained samples P1-P6 have a spherical structure with a pa...
Embodiment 2
[0055] Embodiment 2: Prepare the nano-micelle of wrapping daunorubicin with the A-B-C type triblock polymer of different molecular weights
[0056] Table 2
[0057]
[0058] According to the formula in Table 2, 100 mg of the A-B-C type triblock polymer was weighed and dissolved in acetone, and placed in a round bottom flask. The concentration of the A-B-C type triblock polymer was 10 mg / mL. , evaporate the organic solvent to form a thin and uniform polymer film on the surface of the round bottom flask, and dissolve 5 mg daunorubicin (DNR) in sodium chloride injection to obtain an aqueous solution of daunorubicin. The concentration of ADM is 0.5 mg / mL, the aqueous solution of daunorubicin is added in the round bottom flask, 70 o Hydrate at C for 5 min, filter and sterilize with a 0.22 um microporous membrane to obtain a nanomicelle solution, and freeze-dry to obtain a nanomicelle lyophilized powder loaded with anthracycline antitumor antibiotics. The obtained samples P7-P...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| concentration | aaaaa | aaaaa |
| particle diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com