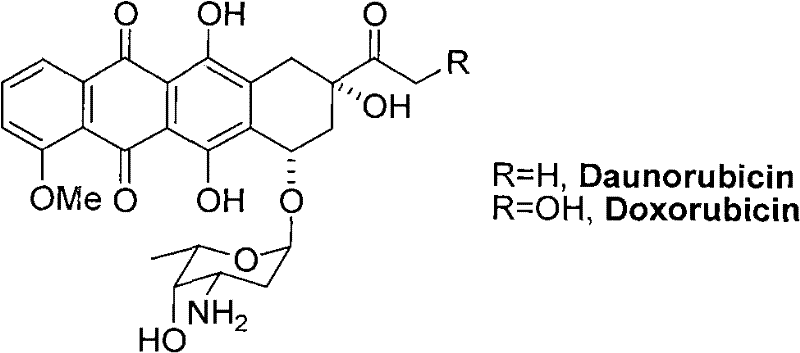
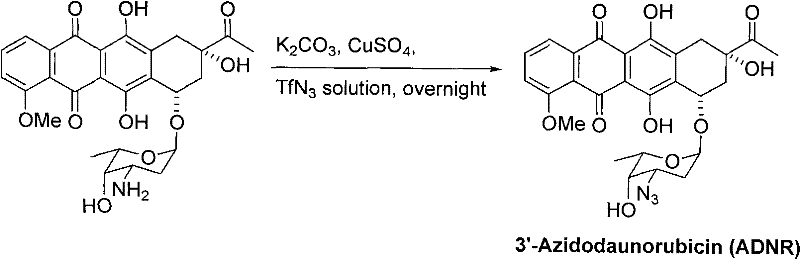
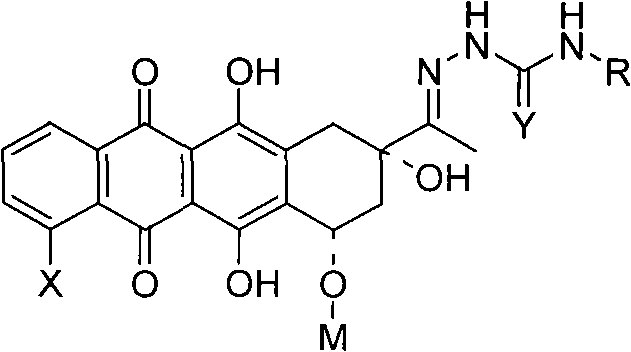
New 3'-azido daunorubicin-13-thiosemicarbazone compound with cancer resistance and preparation method thereof
A compound, phenyl technology, applied in the field of chemistry and medicine, achieves the effect of simple synthesis method and good biological activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0017] 1. Synthesis of substituted thiosemicarbazides I-3 to I-18
[0018]
[0019] Add 50mmol of substituted ammonia, 50mmol of carbon disulfide and 50mmol of triethylamine into the reaction flask, cool to 0°C and react overnight. Wash the reaction solution with ether and dry it for 30 minutes, dissolve the obtained solid in 45 mL of anhydrous chloroform and neutralize 50 mmol of triethylamine, add 50 mmol of ethyl chloroformate dropwise at 0°C, and react for 2 hours. Compounds I-3 to I-18 can be obtained through analysis and purification with yields of 50-80%.
[0020]
[0021] 2.1 Synthesis of 3-substituted hydrazone-3'-azidodaunorubicin (II series compounds)
[0022]
[0023] At room temperature, ADNR (1mmol) and series I compounds (3eq) were added to a 50mL round bottom flask, 10mL absolute ethanol and 1 drop of acetic acid were added, and the reaction process was monitored by TLC. After the reaction, use column chromatography to separate and obtain II series c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com