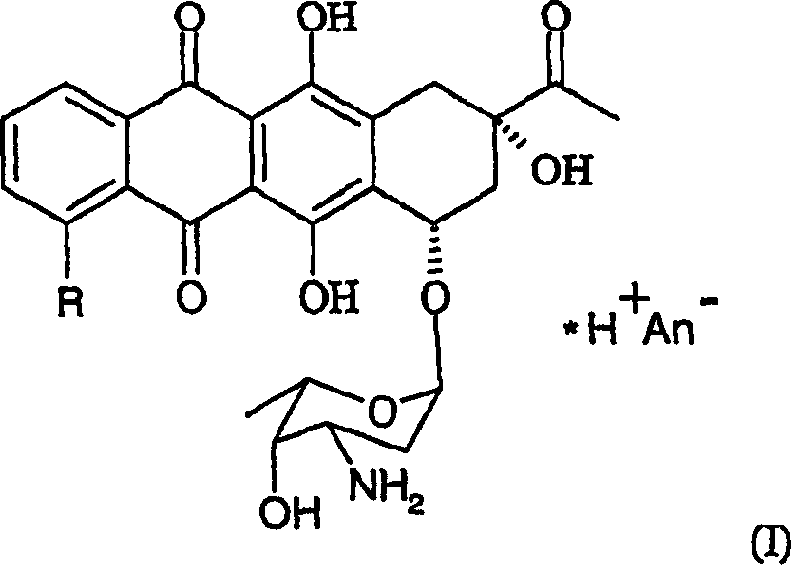
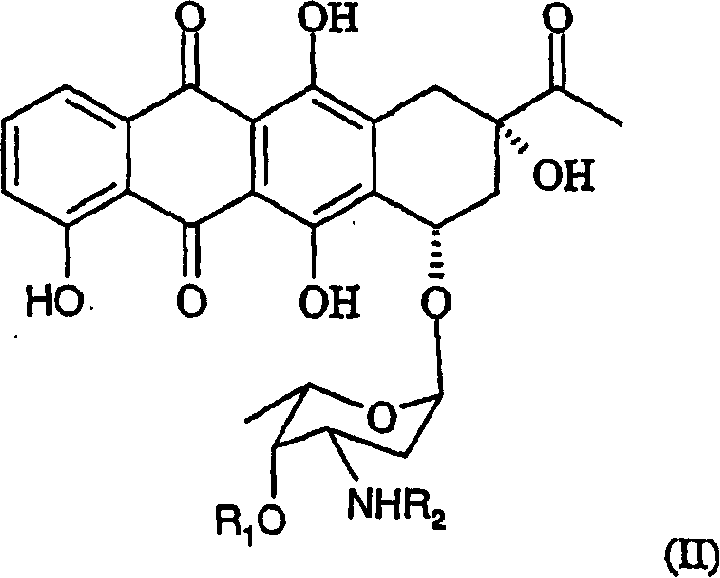
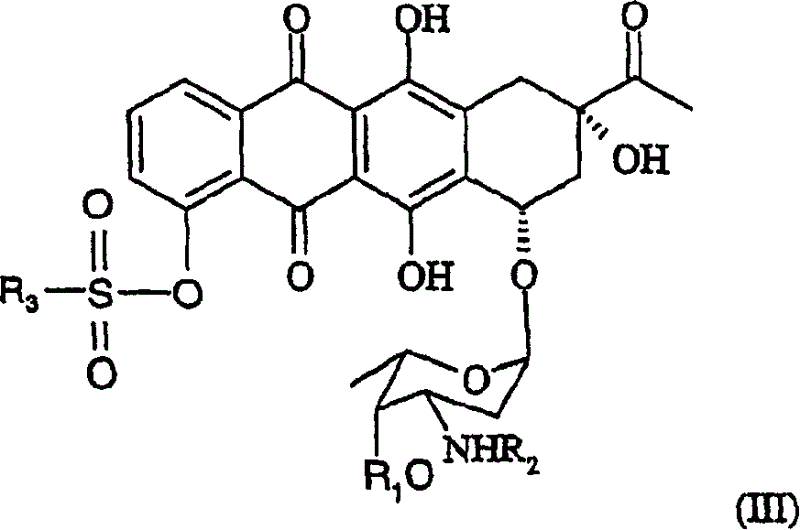
Method of preparing 4-R-substituted 4-demethoxydaunorubicin
A technology of daunorubicin and alkenyl, which is applied in the field of preparation of anthracycline antibiotics, and can solve problems such as complex methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0045] First, 2g of 3’-trifluoroacetamido-4-desmethyldaunomycin (R 1 =H, R 2 = Trifluoroacetyl) dissolved in 0.2 L of pyridine.
[0046] Next, 4 ml of diisopropylethylamine and 0.5 g of 4-dimethylaminopyridine were added to the solution of step (a) in Example 1.
[0047] Next, the solution of step (b) in Example 1 was quenched to 0°C, and 2.5 ml of trifluoromethanesulfonic anhydride that had just been distilled was added.
[0048] Next, the solution of step (c) in Example 1 was incubated for 1 hour at room temperature.
[0049] After incubation, 0.15L concentrated hydrochloric acid, 0.2kg ice and 0.2L dichloromethane were added to the incubation solution.
[0050] Next, the organic layer was washed in 0.2 L of distilled water, and the dichloromethane was removed by evaporation under partial vacuum.
[0051] After evaporation, 1.5 g of 4-trifluoromethanesulfonyl-3'-trifluoroacetamido-4-desmethyldaunorubicin with a purity of 85% (confirmed by HPLC) was prepared.
Embodiment 2
[0054] 1.5g of 4-trifluoromethanesulfonyl-3'-trifluoroacetamido-4-desmethyldaunorubicin (R 1 =H, R 2 = Trifluoroacetyl, R 3 = Trifluoromethyl) dissolved in 0.1 L of dimethylformamide.
[0055] During the stirring process, 2 g of triethylamine formate and 50 mg of palladium acetate were added to the mixture of step (a) in Example 2, and a stream of argon was passed through the mixture.
[0056] Then, the mixture of step (b) in Example 2 was heated to 50°C, and 200 mg of 1,1'-bis(diphenylphosphino)ferrocene was added.
[0057] Then, the mixture of step (c) in Example 2 was heated at 50°C for 8 hours.
[0058] Then, the mixture of step (d) in Example 2 was poured into water under vigorous stirring to form a precipitate (4-demethoxy-3'-trifluoroacetamidodaunorubicin).
[0059] The precipitate (4-demethoxy-3'-trifluoroacetamidodaunorubicin) was filtered, and then purified by preparative chromatography.
[0060] The output of this method is 0.8-0.85 g of 4-demethoxy-3'-trifluoroacetamid...
Embodiment 3
[0062] 0.85 g of 4-demethoxy-3'-trifluoroacetamido daunorubicin was added to a stirred aqueous solution of 0.1N NaOH (0.06 L) and kept at 30°C for 30 minutes. The color of the solution became dark blue-purple.
[0063] Then, under vigorous stirring, the reaction mixture was poured into 0.5 L of a 10-12% solution of chloroform in butanol (heated to 40°C).
[0064] Then, under vigorous stirring, hydrochloric acid (1:3) was added to the mixture and titrated to a pH of 8.8-9.0.
[0065] Then, the obtained organic layer was washed in distilled water.
[0066] Then, 0.1 L of distilled water was added to the organic layer washed in step (d) of Example 3, and 0.8N hydrochloric acid was added, and the pH was titrated to 3.5. The solution of step (e) in Example 3 was stirred vigorously, and the aqueous layer containing 4-demethoxydaunorubicin hydrochloride (idarubicin) was separated. The idarubicin hydrochloride solution was evaporated to 50% of its original volume and purified by chromatog...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com