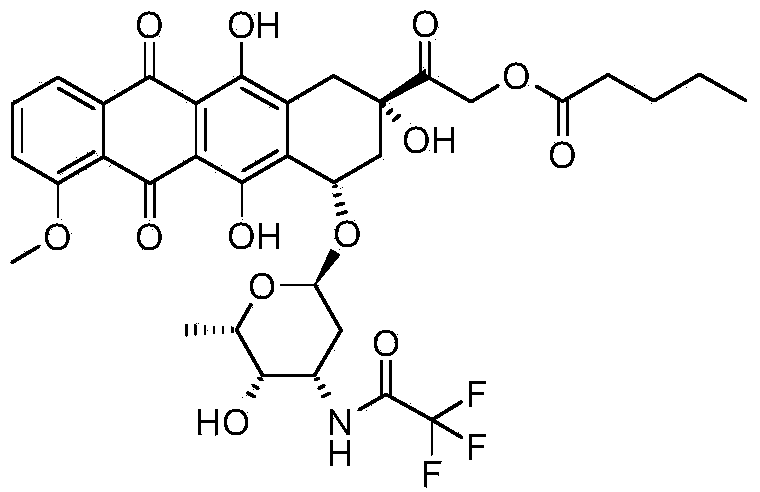
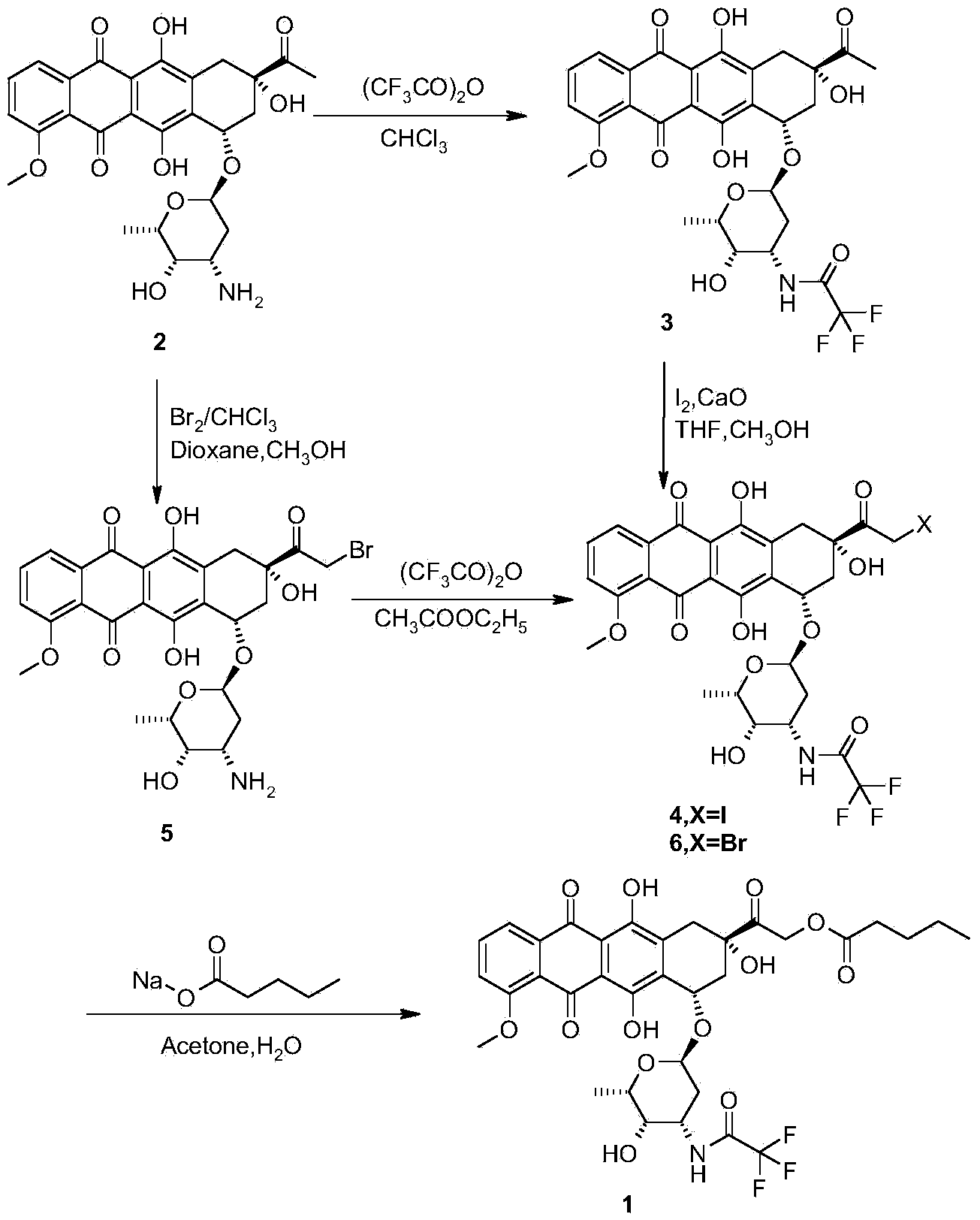
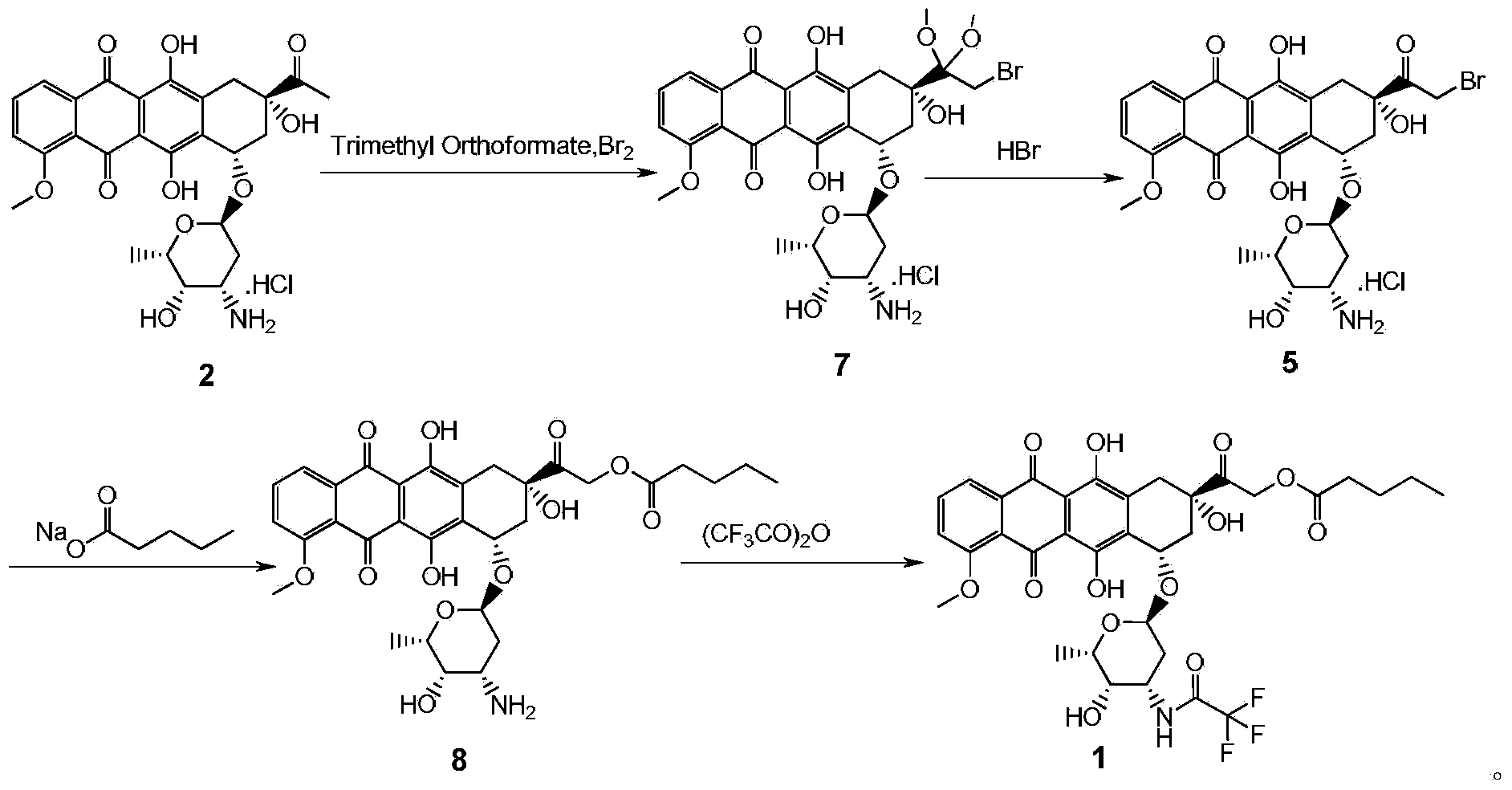
Synthesis method for valrubicin
A synthetic method, the technology of valrubicin, which is applied in the field of drugs for the treatment of BCG refractory bladder carcinoma in situ, can solve the problem of unstable polybrominated and bicinoid compounds, which are not suitable for industrial production of high-purity raw materials Medicine and other problems, to achieve the effect of high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Embodiment 1: the preparation of compound 7
[0024] Add daunorubicin hydrochloride (15.0g, 26.6mmol) into a 2L three-necked flask, then add 150ml of methanol and 300ml of 1,4-dioxane, stir and cool to 8±2°C under nitrogen protection, then add orthoformic acid Trimethyl ester (13.0g, 122mmol); bromine (5.1g, 31.9mmol) was diluted with about 15ml of dichloromethane and added dropwise to the reaction flask at 8±2°C. After dropping, the solution was stirred and reacted at 8±2°C under the protection of nitrogen for 2 to 3 hours, then quenched by adding propylene oxide (2.32g, 39.9mmol), and cooled to 0±2°C, adding 1350ml of isopropyl ether dropwise. Stir and crystallize at 0±2°C for 1 to 2 hours, suction filter under nitrogen protection, rinse the filter cake with an appropriate amount of isopropyl ether, drain it, and dry it in vacuum at room temperature for 2 to 4 hours to obtain compound 7 as a red solid powder with a yield of 18.0 g. The yield was 98.3%, and the produc...
Embodiment 2
[0025] Embodiment 2: the preparation of compound 5
[0026] Add compound 7 (20.0g, 29.0mmol) to a 5L three-necked flask, add 1000ml of acetone, stir and cool to 0±2°C under nitrogen protection, dilute 40% HBr (5.9g, 29.0mmol) with 10ml of water and dilute at 0 Add it dropwise to the reaction flask at ±2°C; after the drop is complete, the solution is stirred and reacted overnight at 0±2°C under the protection of nitrogen. The next day, add 2500ml of isopropyl ether dropwise, stir and crystallize at 0±2°C for 1-2 hours, suction filter under nitrogen protection, rinse the filter cake with an appropriate amount of isopropyl ether, drain it, and dry it in vacuum at room temperature for 2-4 hours to obtain Red solid powder compound 5, yield 18g, yield 96.5%, purity 95.1% (HPLC, area normalization method).
Embodiment 3
[0027] Embodiment 3: the preparation of compound 8
[0028] Add compound 5 (15.0g, 23.3mmol), sodium n-valerate (7.2g, 58.3mmol), 750ml of acetone and 7.5ml of water into a 1L three-necked flask, stir and heat to 60±2°C under nitrogen protection, and place at 60 Stir the reaction at ±2°C for 2-3 hours. Suction filtration while hot, the filtrate was concentrated to dryness under reduced pressure at 25±3°C, the concentrate was separated and purified by silica gel normal phase column chromatography, and eluted with dichloromethane:methanol=10:1 as the eluent, the collected purity was greater than 95% The eluate was concentrated to dryness under reduced pressure at 25±3°C to obtain red solid compound 8, yield 8.0g, yield 54.8%, purity 96.5% (HPLC, area normalization method); MS / ES+: (M+ h) + 628.28.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com