Cytarabine prodrug derivatives and purposes thereof in resisting cancers and tumors
A technology of cytarabine and its derivatives, which is applied in the medical field and can solve problems such as toxicity, slowness, and inability to treat liver cancer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
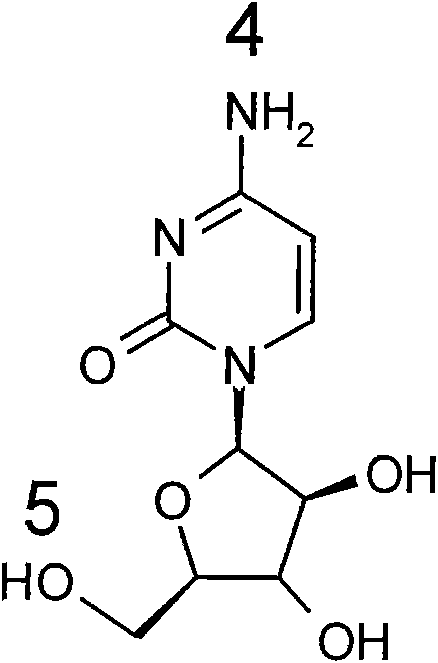
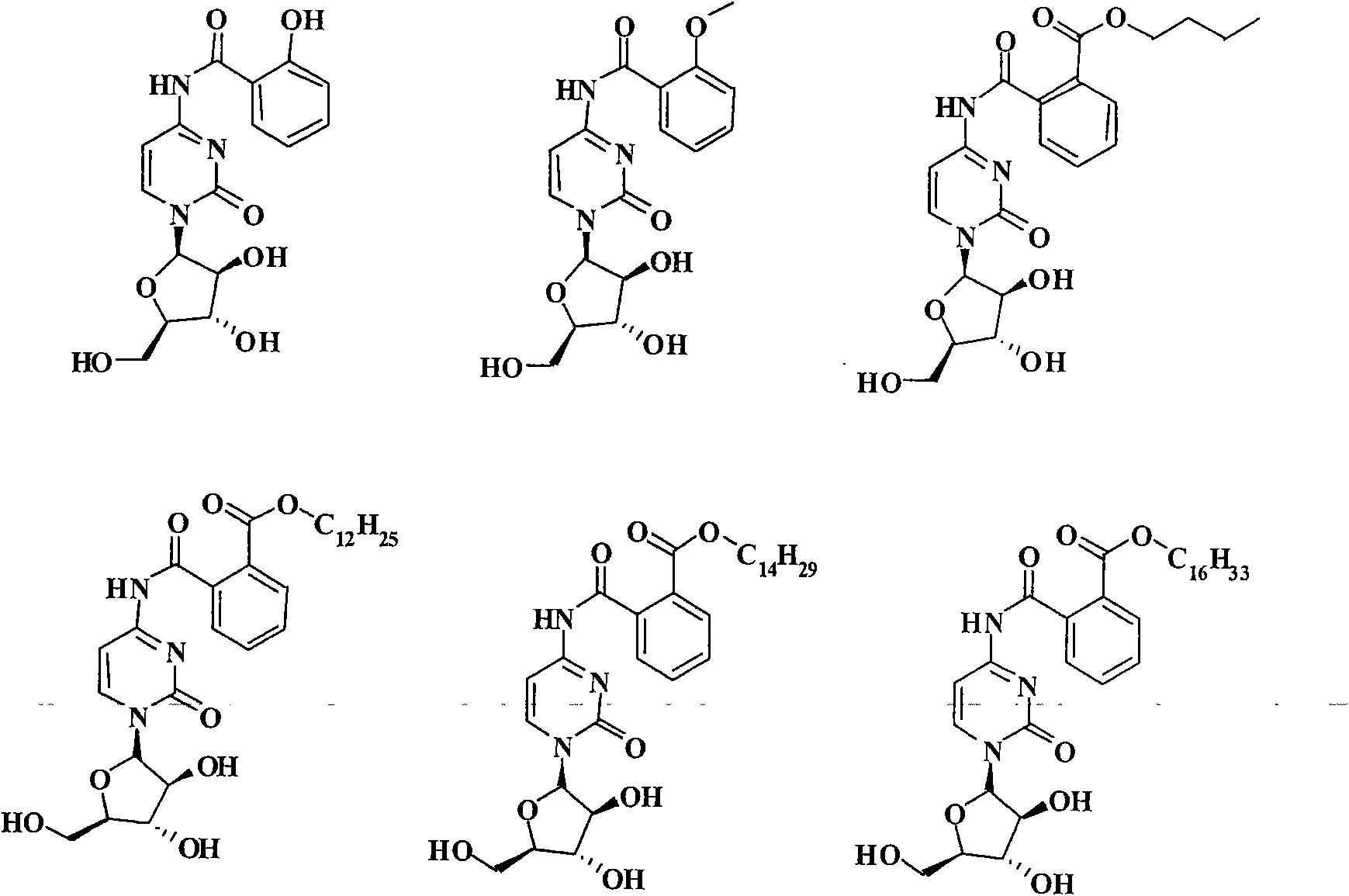
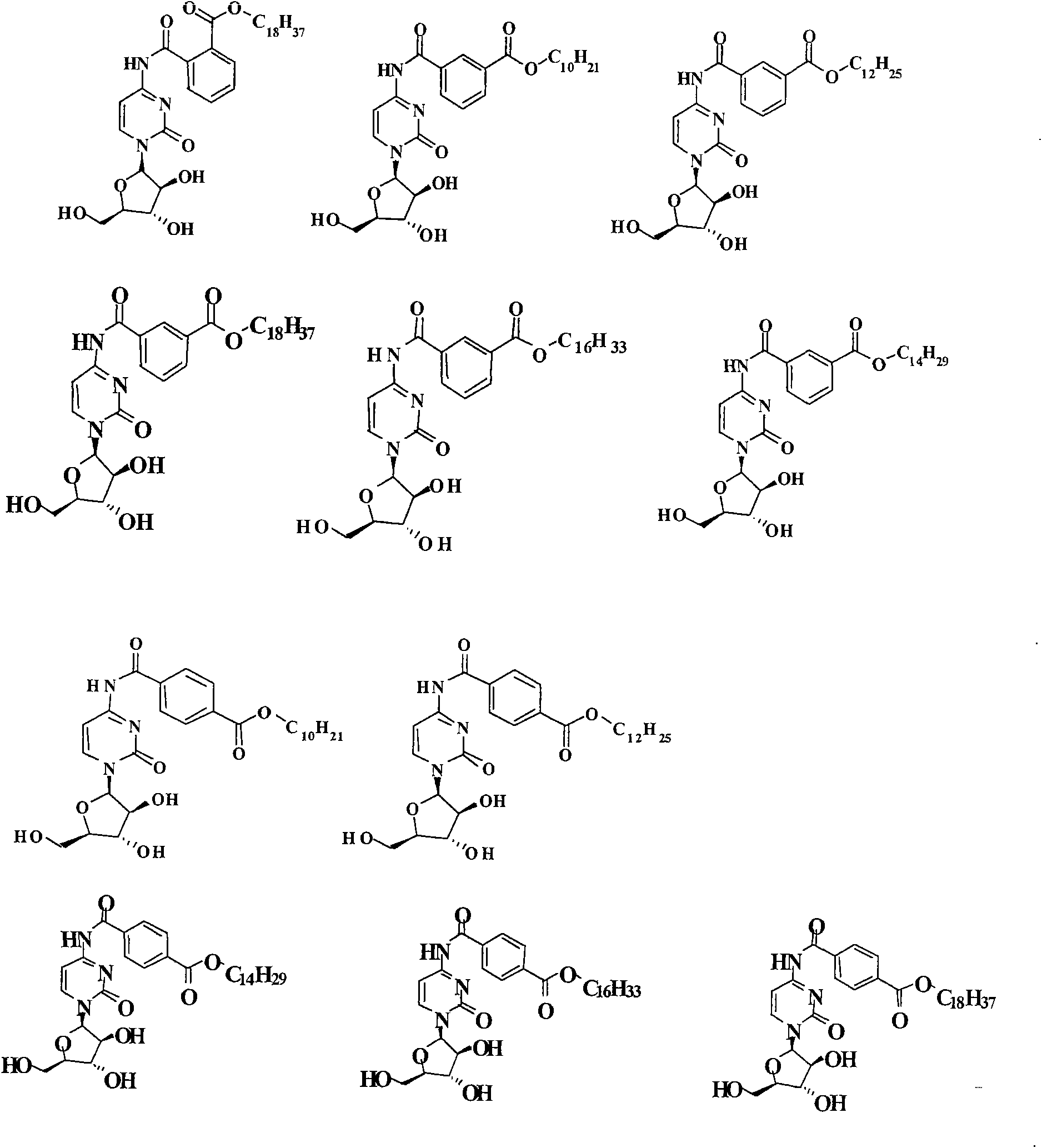
[0071] The synthetic routes of some representative cytarabine derivatives of the present invention are listed below. Other similar cytarabine derivatives of the present invention are synthesized by the same or similar method.
[0072] Synthetic route 1:
[0073]
[0074] Synthesis of cytarabine derivative 1 (Scheme 1): cytarabine (1.0 g, 4 mmol) and acetic anhydride (0.9 ml, 4.8 mmol) were dissolved in methanolic MeOH (500 ml). The reaction mixture was heated to reflux for 4 hours. The reaction solution was purified by column chromatography (silica gel, developer: dichloromethane / methanol=10 / 1) to obtain cytarabine derivative 1 (84.6 mg), with LC (UV 254nm) purity>95%. LC-MS m / z 286[M+H] + (molecular formula C 11 h 15 N 3 o 6 , molecular weight 285). 1 H NMR (600MHz, DMSO-d 6 )δ10.84(s, 1H), 8.06(d, 1H), 7.18(d, 1H), 6.05(d, 1H), 5.50(d, 1H), 5.49(d, 1H), 5.06(t, 1H ), 4.06(m, 1H), 3.94(m, 1H), 3.83(m, 1H), 3.61(t, 2H), 2.10(t, 3H).
[0075] Synthetic route 2:
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com