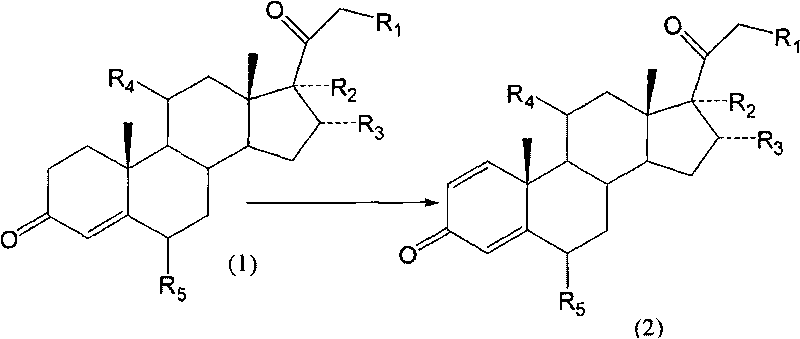
Biological dehydrogenation preparation method of steroid drug intermediate
A technology of steroidal drugs and intermediates, which is applied in the field of biological dehydrogenation preparation of pregnant drug intermediates, can solve the problems of prolonging reaction time, reducing conversion rate, and producing dehydrogenation products, so as to shorten reaction time and improve conversion rate, the effect of overcoming toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1-1
[0049] Example 1-1: Using 6α-methyl-11β,17α-dihydroxy-pregn-4-ene-3,20-dione as a substrate
[0050] The simple Arthrobacter (AS 1.94*) was cultured in inclined plane, primary culture and secondary culture in sequence. The culture temperature was 30°C. The micronized substrate was put into a 5L fermentor with a feed concentration of 4% and a reaction temperature of 30 ℃. The reaction time is 48 hours. After the reaction is completed, the temperature is raised to 70° C. to terminate the reaction. The fermentation broth is extracted with ethyl acetate, and the organic phase is concentrated. The substrate conversion rate is measured to be 71.3%.
Embodiment 1-2
[0052] Using the substrate and fermentation process of Example 1-1, adding 150ml Tween-80 as a solvent during feeding, the reaction time was shortened to 36 hours, the fermentation broth was extracted with ethyl acetate, and the organic phase was concentrated. The product conversion rate was 76.2%.
Embodiment 2
[0053] Example 2: Using 6α-methyl-17α,21-dihydroxy-pregn-4-ene 3,11,20-trione-21-acetate as a substrate;
[0054] The simple Arthrobacter (AS 1.754) was cultured in slope, primary culture and secondary culture in sequence. The culture temperature was 31℃. The micronized substrate was put into a 5L fermentor with a feed concentration of 2% and a reaction temperature of 32℃. . The reaction time is 42 hours. After the reaction is completed, the temperature is raised to 70° C. to terminate the reaction. The fermentation broth is extracted with ethyl acetate, and the organic phase is concentrated. The measured substrate conversion rate is 75.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com