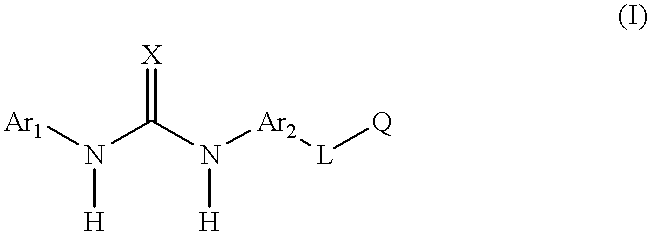
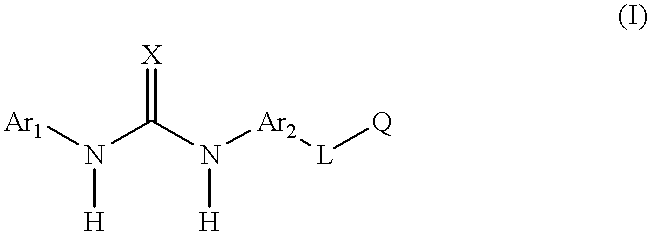
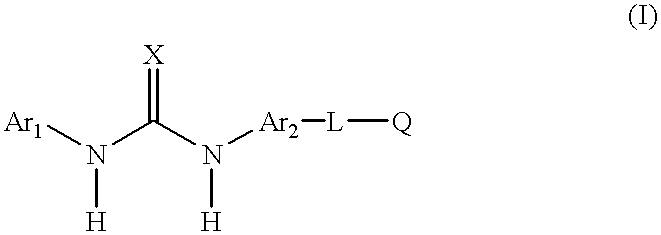Aromatic heterocyclic compounds as antiinflammatory agents
a heterocyclic compound and antiinflammatory agent technology, applied in the field of aromatic heterocyclic compounds as antiinflammatory agents, can solve the problems of bioavailability and stability, high cost of protein therapeutics, etc., and achieve the effects of improving stability of inhibitors, facilitating the administration of pharmaceutical compositions, and increasing dissolution or dispersion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
1-[5-tert-Butyl-2-p-tolyl-2H-pyrazol-3-yl]-3-[4-(2-morpholin-4-yl-ethoxy)n-aphthalen-1-yl]-urea:
[0286] 36
[0287] A mixture of 4-methylphenyl hydrazine hydrochloride (10.0 g) and 4,4-dimethyl-3-oxopentanenitrile (8.67 g) in 150 mL ethanol and 7 mL concentrated HCl was heated at reflux overnight, cooled to room temperature, basified to pH 12 with alkali and extracted with diethyl ether. The combined organic extracts were washed with brine and dried (MgSO.sub.4). Removal of the volatiles in vacuo left a residue which was triturated with hot petroleum ether (100 mL) and provided 12.5 g of LXVII.
[0288] To a mixture of 4-amino-1-naphthol hydrochloride (LXIII) (172.1 g) in 750 mL anhydrous THF at -78.degree. C. was added dropwise over 60 min n-butyl lithium (490 mL of a 1.60 M solution in hexanes). After the addition was complete the mixture was allowed to warm to room temperature and then cooled to -78.degree. C. and di-tert-butyl dicarbonate ((BOC).sub.2O, 192 g) in 200 mL THF was added o...
example 2
1-[5-tert-Butyl-2-p-tolyl-2H-pyrazol-3-yl]-3-[4-(3-(tetrahydropyran-2-yl-o-xy)propyn-1-yl)naphthalen-1-yl]-urea:
[0291] 37
[0292] Tetrahydro-2-(2-propynyloxy)-2H-pyran (LXVIII) (2.50 mL; 17.8 mmol) in 100 mL anhydrous THF at -78.degree. C. under inert atmosphere was treated with n-butyllithium (7.1 mL of a 2.5 M solution in hexanes), added via syringe. The reaction was warmed to -20.degree. C. and after 1 h stirring, tributyltin chloride (4.8 mL, 17.8 mmol) was added. After stirring at -20.degree. C. for 1 h the reaction mixture was quenched with dilute NaHCO.sub.3 solution (75 mL) and extracted with ethyl ether (3.times.50 mL). The combined ethereal extracts were washed with brine and dried (MgSO.sub.4). After filtration all volatiles were removed in vacuo to produce LXIX as a yellow oil (4.7 g; 11.0 mmol or 62% yield).
[0293] A mixture LXVII (Example 1) (1.00 g; 3.76 mmol) and phosgene (5.6 mL of a 2 M solution in toluene) and 4-bromonaphthylamine were reacted according to Method B (...
example 3
1-[5-tert-butyl-2-p-tolyl-2H-pyrazol-3-yl]-3-[4-(3-pyridin-4-yl-propoxy)na-phthalen-1-yl]-urea (3):
[0295] 38
[0296] To a mixture of LXIV (Example 1) (0.51 g), 4-pyridinyl-1-propanol (0.76 mL), and triphenylphosphine (1.5 g) in 10 mL anhydrous THF was added dropwise diethyl azodicarboxylate (DEADC, 0.90 mL). After stirring overnight, the volatiles were removed in vacuo. Purification of the residue by flash chromatography using 25% hexanes in ethyl acetate as the eluent and concentration of the product-rich fractions in vacuo provided ether LXXI. A mixture of LXXI (0.74 g) and HCl (5 mL, 4.0 M in dioxane) in 10 mL anhydrous dioxane was stirred overnight. Collection of the precipitate by vacuum filtration provided LXXII. LXXVII (Example 1) (0.23 g), saturated NaHCO.sub.3 (15 mL), dichloromethane (15 mL), phosgene (2.1 mL, 1.93M in toluene) and LXXII (0.32 g) were reacted according to Method B (Scheme I and Example 1). Purification of the residue by flash chromatography using 25% hexanes...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Electric charge | aaaaa | aaaaa |
| Composition | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com