Lamivudin stearate and synthesis method and application
A technology of lamivudine stearate and lamivudine, which is applied in the field of drug synthesis, can solve problems such as difficult loading and limited drug loading capacity, and achieve the effects of increasing drug concentration, reducing distribution, and increasing uptake
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] Embodiment 1: the synthesis of the prodrug lamivudine stearate of lamivudine
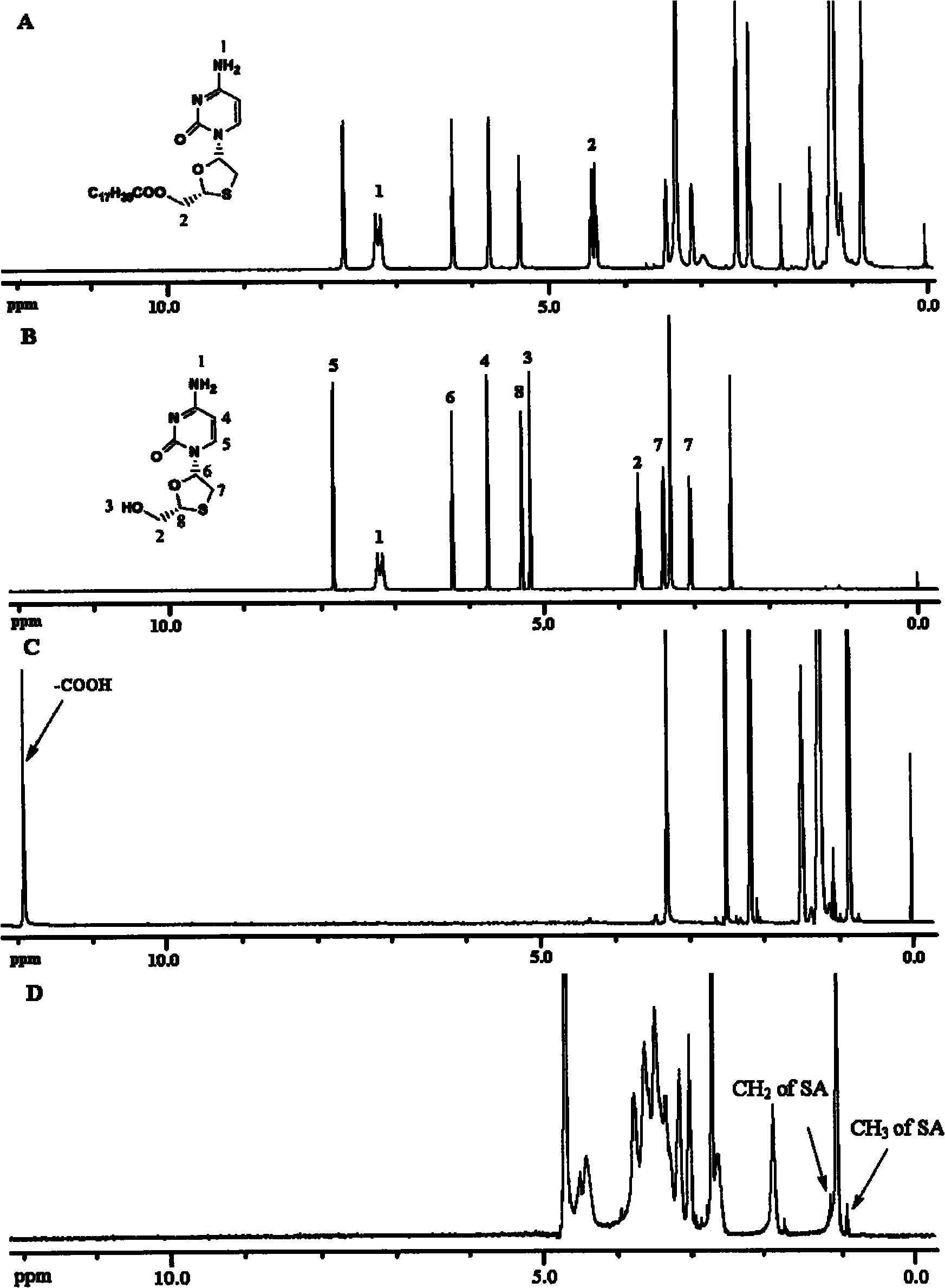
[0023] Accurately weighed dry 1.29g lamivudine, 2.778g dicyclohexylcarbodiimide and 142mg 4-dimethylaminopyridine were dissolved in 50mL anhydrous dichloromethane; accurately weighed 3.18g stearic acid Dissolve in 50mL of anhydrous dichloromethane. Under the condition of 400rpm magnetic stirring, the stearic acid organic solution was added dropwise to the mixed organic solution of lamivudine, and heated to reflux at 30° C. for 4 days under nitrogen protection. After the reaction was completed, the solvent dichloromethane was distilled off to obtain a crude product. Using methanol / dichloromethane (1:20, v / v) as eluent, the crude product was separated and purified by silica gel column chromatography to obtain the target product lamivudine stearate (I). figure 1 It is the nuclear magnetic resonance spectrum of lamivudine stearate, lamivudine, and stearic acid, and the synthesis of lamivudine s...
Embodiment 2
[0024] Embodiment 2: the preparation of lamivudine stearate chitosan stearic acid micelles
[0025] 1) Synthesis of chitosan stearic acid graft
[0026] Weigh 2.0g of chitosan oligosaccharide with a molecular weight of 1.8KDa, add 50mL of deionized water and stir to dissolve; and weigh 0.8g of stearic acid and 5.5g of carbodiimide and dissolve in 40ml of ethanol. The chitosan oligosaccharide solution was heated to 80° C., and the stearic acid solution was added dropwise with stirring. The reaction temperature was maintained at 80° C., and the reaction was performed for 5 h under the condition of magnetic stirring at 400 rpm. The final reaction solution was placed in a dialysis bag (molecular weight: 7 kDa), and dialyzed against deionized water for 24 hours to remove residual carbodiimide and reaction by-product isourea. The dialysate was freeze-dried to obtain chitooligosaccharide stearic acid graft. figure 1 D is the H NMR spectrum of the chitosan stearic acid graft.
[0...
Embodiment 3
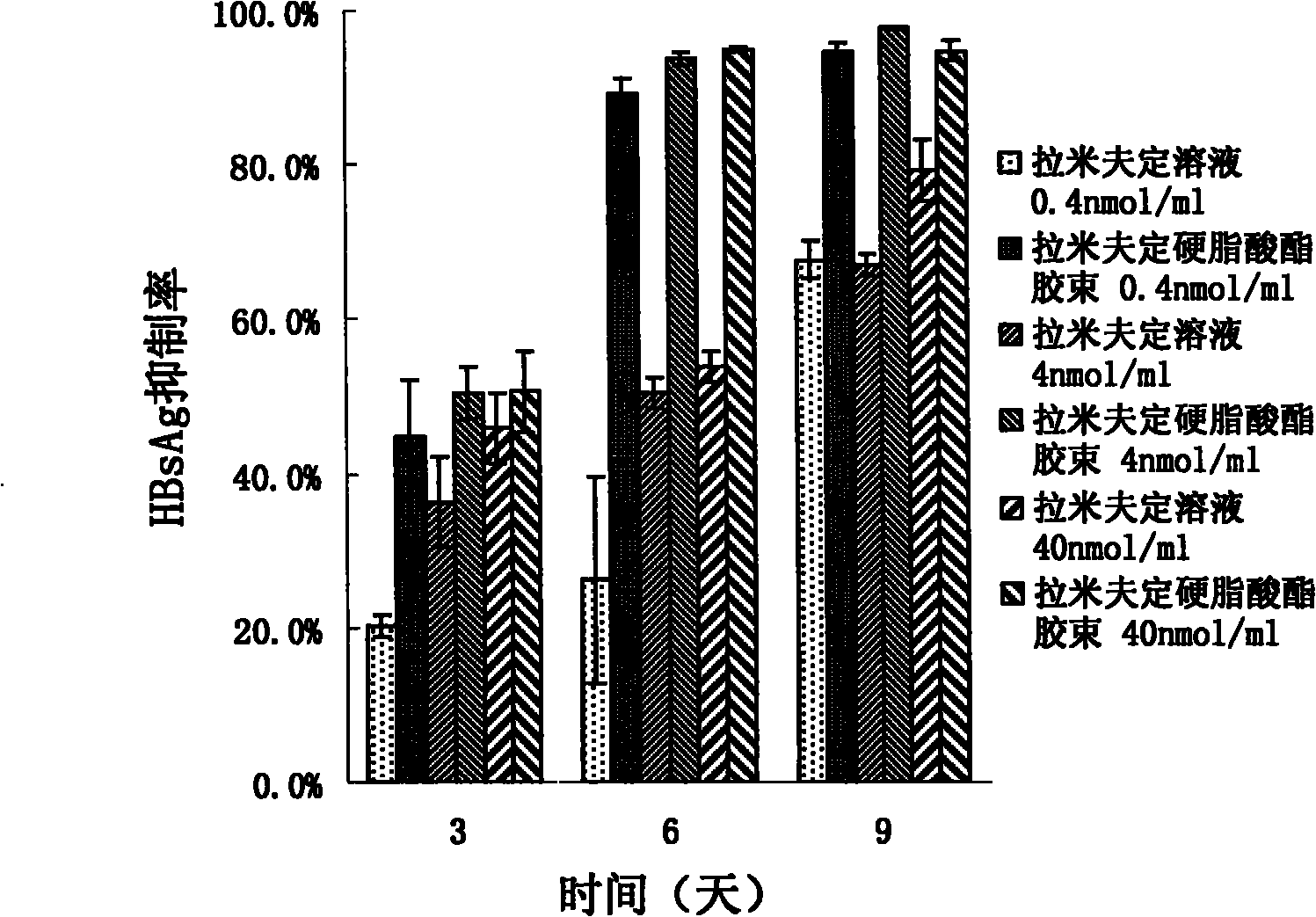
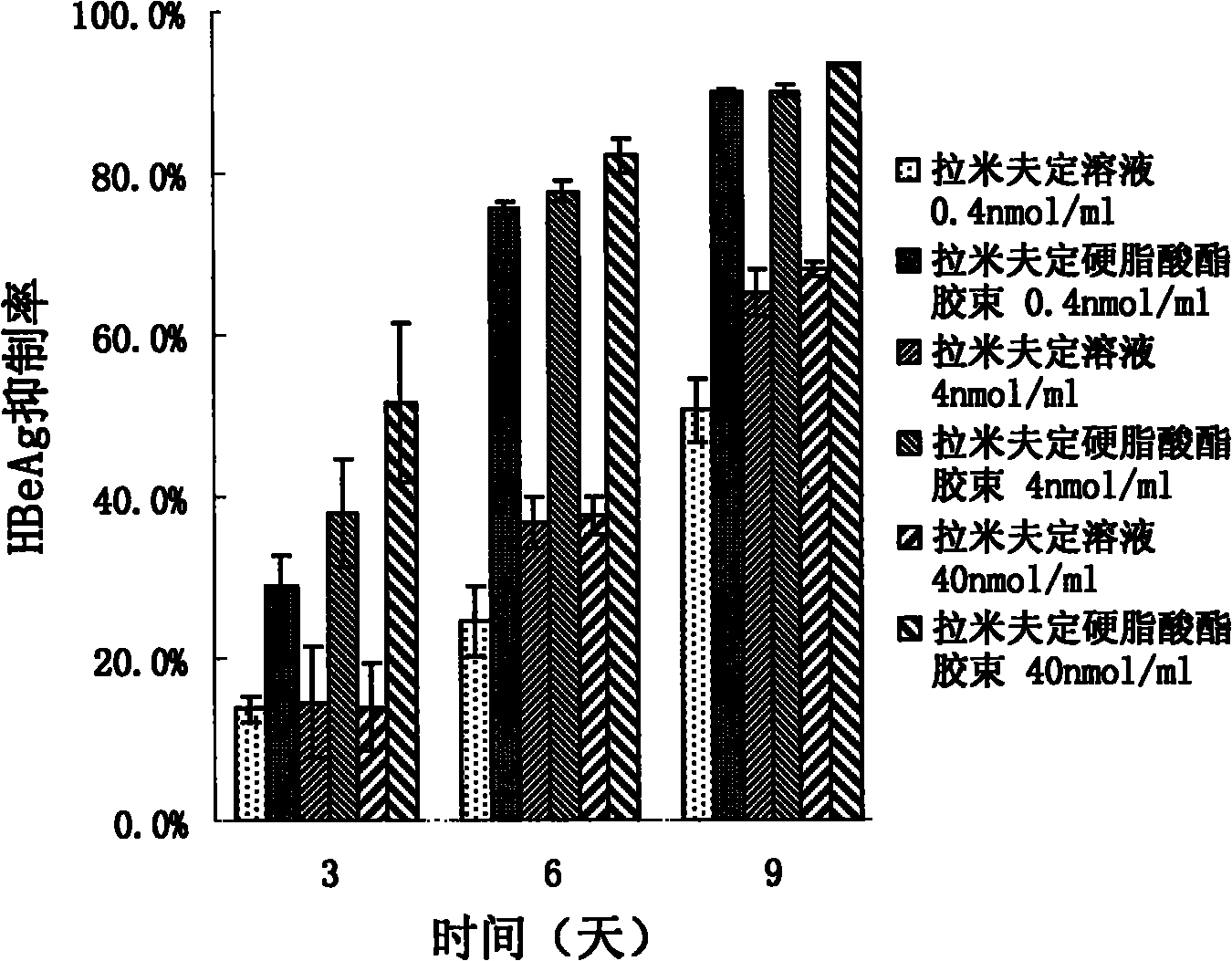
[0036] Embodiment 3: the preparation of the graft micelles of loading lamivudine stearate
[0037] 1) Synthesis of chitosan stearic acid graft
[0038] Weigh 2.0g of chitosan oligosaccharide with a molecular weight of 1.8KDa, add 50mL of deionized water and stir to dissolve; and weigh 0.8g of stearic acid and 5.5g of carbodiimide and dissolve in 40ml of ethanol. The chitosan oligosaccharide solution was heated to 80° C., and the stearic acid solution was added dropwise with stirring. The reaction temperature was maintained at 80° C., and the reaction was performed for 5 h under the condition of magnetic stirring at 400 rpm. The final reaction solution was placed in a dialysis bag (molecular weight: 7 kDa), and dialyzed against deionized water for 24 hours to remove residual carbodiimide and reaction by-product isourea. The dialysate was freeze-dried to obtain chitooligosaccharide stearic acid graft. figure 1 D is the H NMR spectrum of the chitosan stearic acid graft.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com