Lamivudine tablet and preparation method thereof
A lamivudine tablet and lamivudine technology are applied in the directions of antiviral agents, pharmaceutical formulations, medical preparations with non-active ingredients, etc., and can solve the problem that diseases cannot be effectively and timely treated, tablet hardness is reduced, problems such as poor tablet stability, to achieve the effect of ensuring drug efficacy, rapid dissolution, and good compatibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
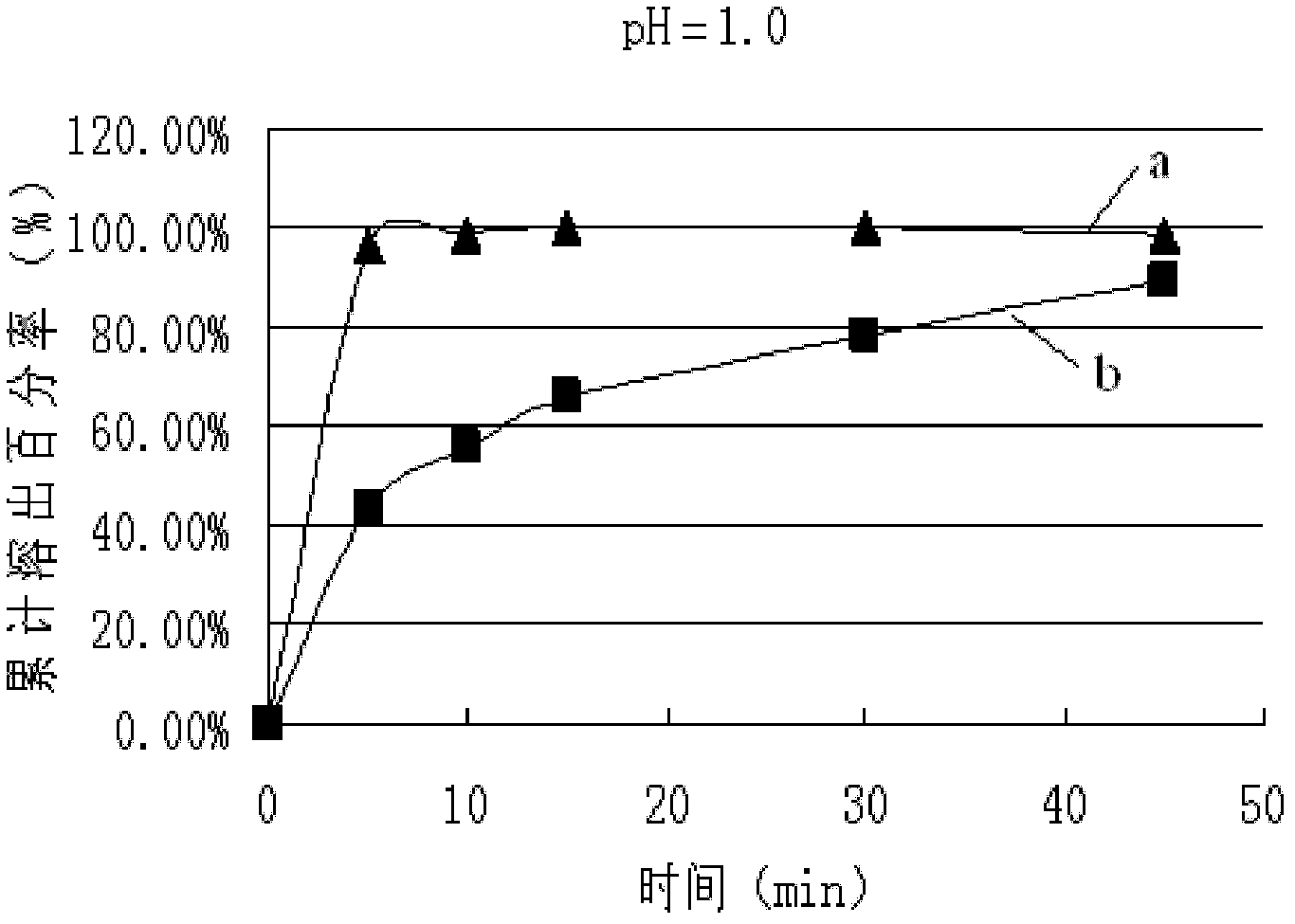
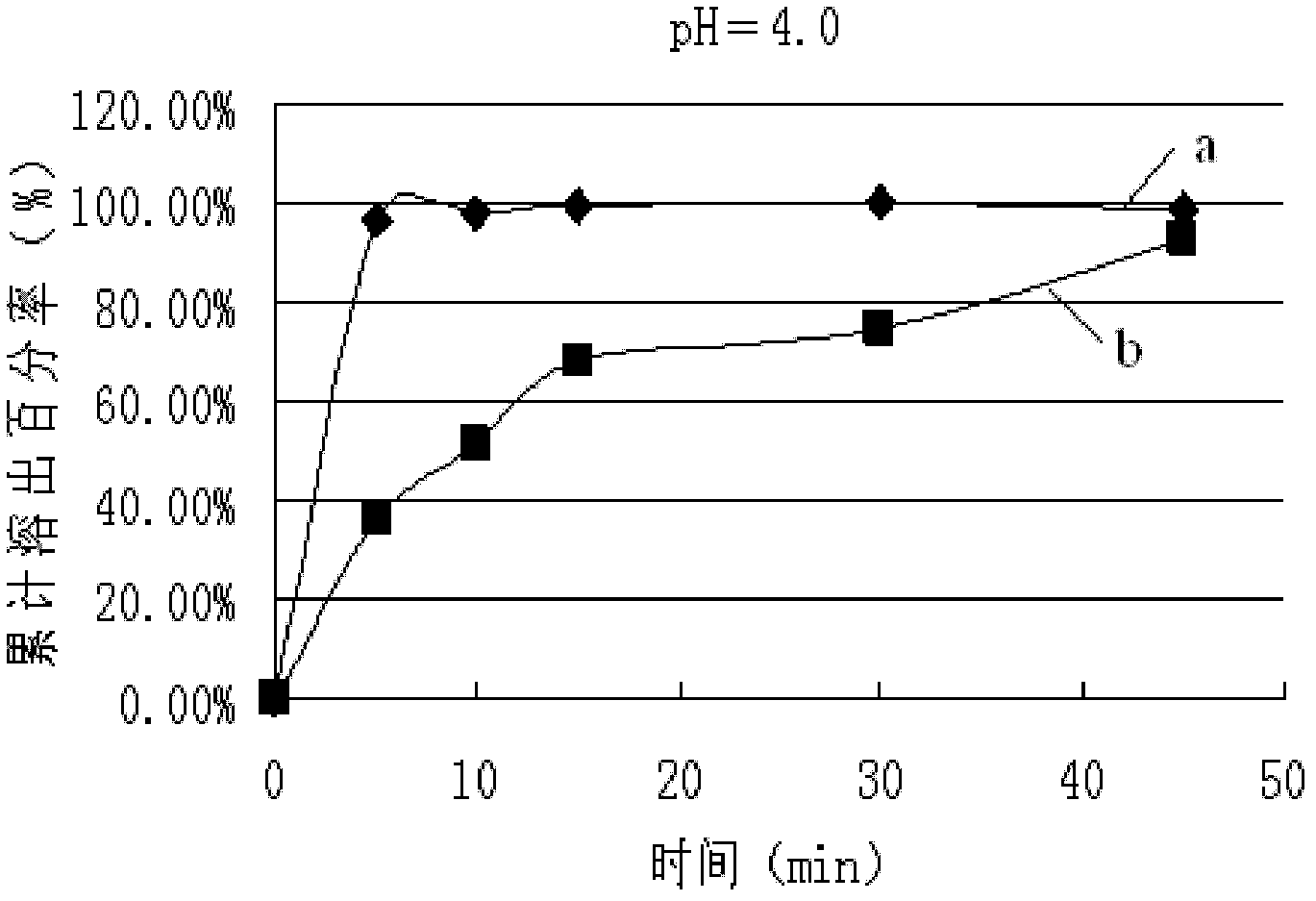
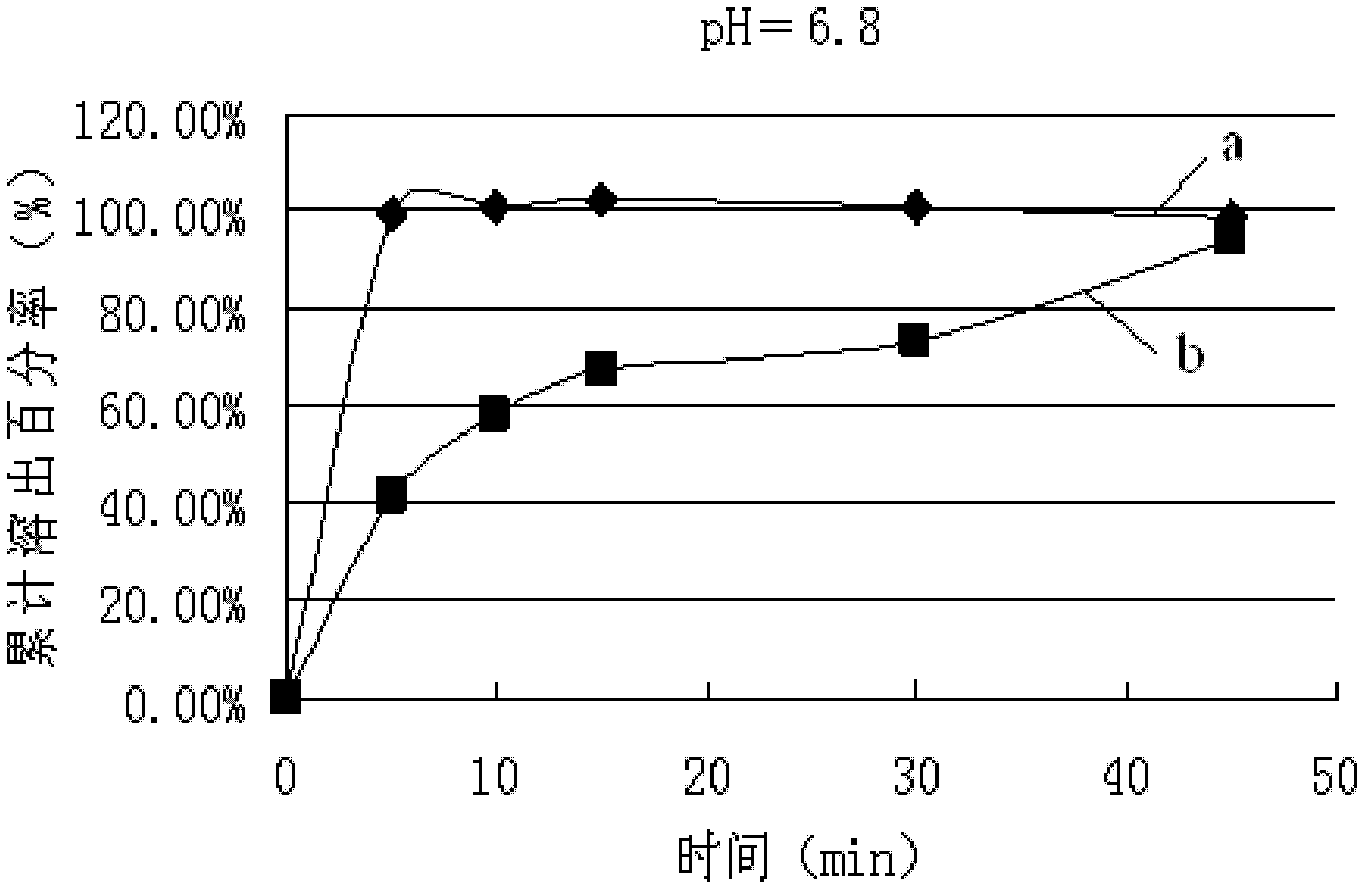
Image
Examples
Embodiment 1
[0041] This embodiment is used to illustrate the preparation method of the lamivudine tablet provided by the invention.
[0042] Tablet core raw materials: lamivudine 1000g, compressible microcrystalline cellulose 1100g, sodium carboxymethyl starch 100g, silicon dioxide 30g, magnesium stearate 20g
[0043] Film coating raw materials: polyvinyl alcohol 80g, Tween 80 10ml, titanium dioxide 10g, water 1000ml
[0044] Preparation:
[0045] (1) Get the compressible microcrystalline cellulose, sodium carboxymethyl starch, and silicon dioxide of the above weight parts, and cross a 65-mesh sieve to obtain a tablet filler;
[0046] (2) get the lamivudine of above-mentioned weight portion, cross 65 mesh sieves, mix with tablet filler uniformly to obtain compound;
[0047] (3) Add the above-mentioned magnesium stearate by weight before tableting, mix evenly with the mixture, put it on the tablet press and press it into tablets, 10000 tablets can be prepared, each tablet contains 100mg ...
Embodiment 2
[0051] This embodiment is used to illustrate the preparation method of the lamivudine tablet provided by the invention.
[0052] Tablet core raw materials: lamivudine 500g, compressible microcrystalline cellulose 600g, sodium carboxymethyl starch 36g, silicon dioxide 10g, magnesium stearate 7.5g
[0053] Film coating raw materials: Colorcon moisture-proof gastric soluble coating powder 90g, water 800ml
[0054] Preparation:
[0055] Using the preparation method of Example 1, 5000 lamivudine tablets can be prepared, each containing 100 mg of active ingredient.
Embodiment 3
[0057] This embodiment is used to illustrate the preparation method of the lamivudine tablet provided by the invention.
[0058] Tablet core raw materials: lamivudine 1000g, compressible lactose 1300g, sodium carboxymethyl starch 30g, cross-linked polyvinylpyrrolidone 40g, talcum powder 17.5g
[0059] Film coating raw materials: hydroxypropyl methylcellulose 30g, Tween 8015ml, 2% lemon yellow aqueous solution 5ml, 1% carmine aqueous solution 3ml, absolute ethanol 500ml and water 500ml
[0060] Preparation:
[0061] Using the preparation method of Example 1, 10,000 lamivudine tablets can be prepared, each containing 100 mg of active ingredient.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com