Daucane type sesquiterpenes and preparation method and application thereof
A technology of carotane and sesquiterpene, which is applied in the field of preparation of anti-HBV drugs, new carotane-type sesquiterpene compounds and its preparation, and can solve the problems that patients cannot be cured
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
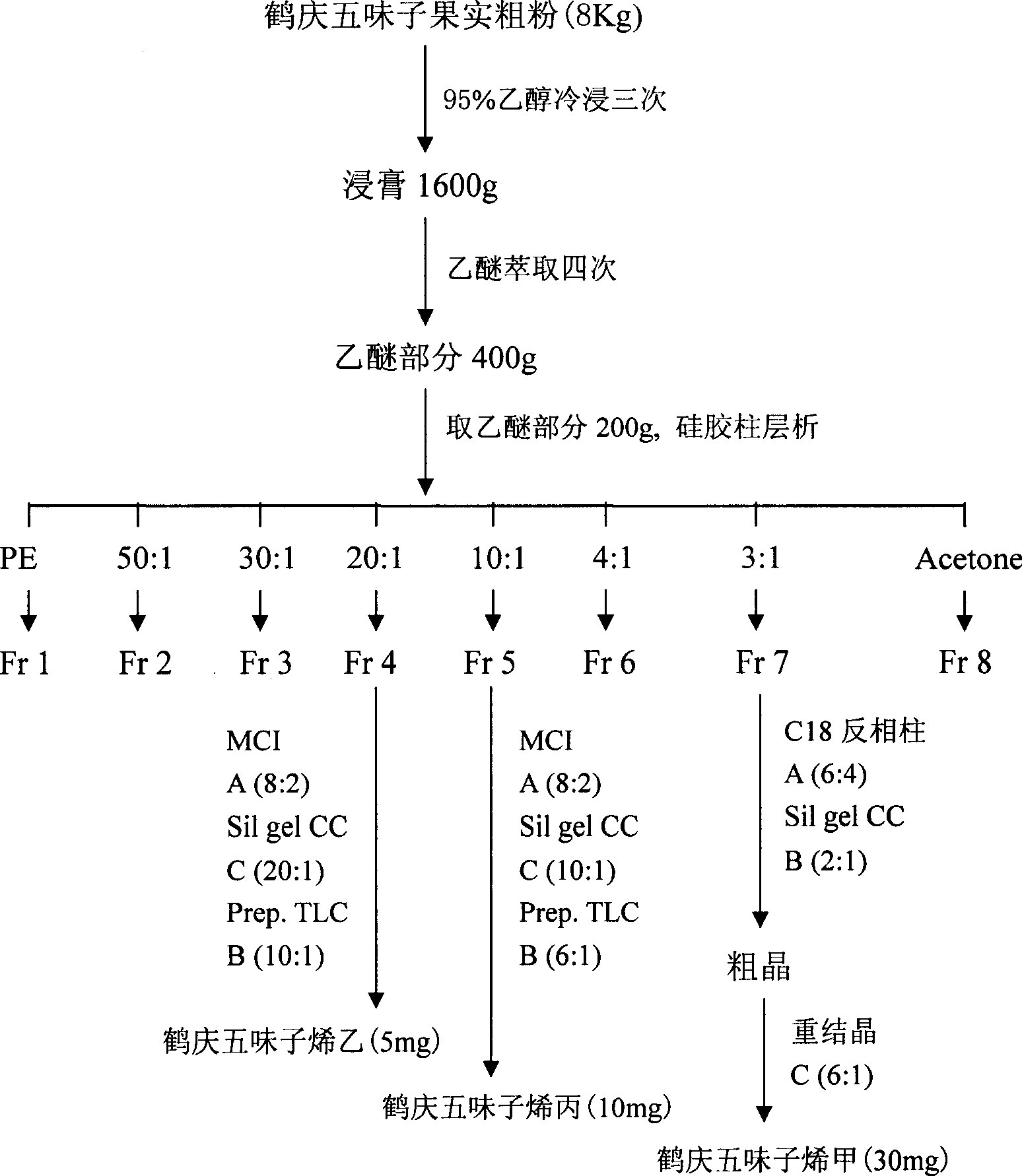
[0022] Example 1 Preparation of Heqing Schisandrin A-C
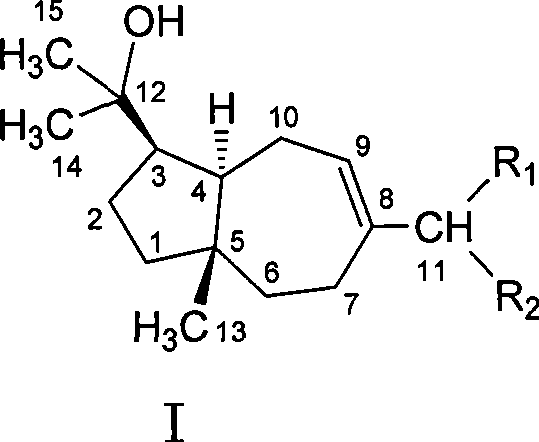
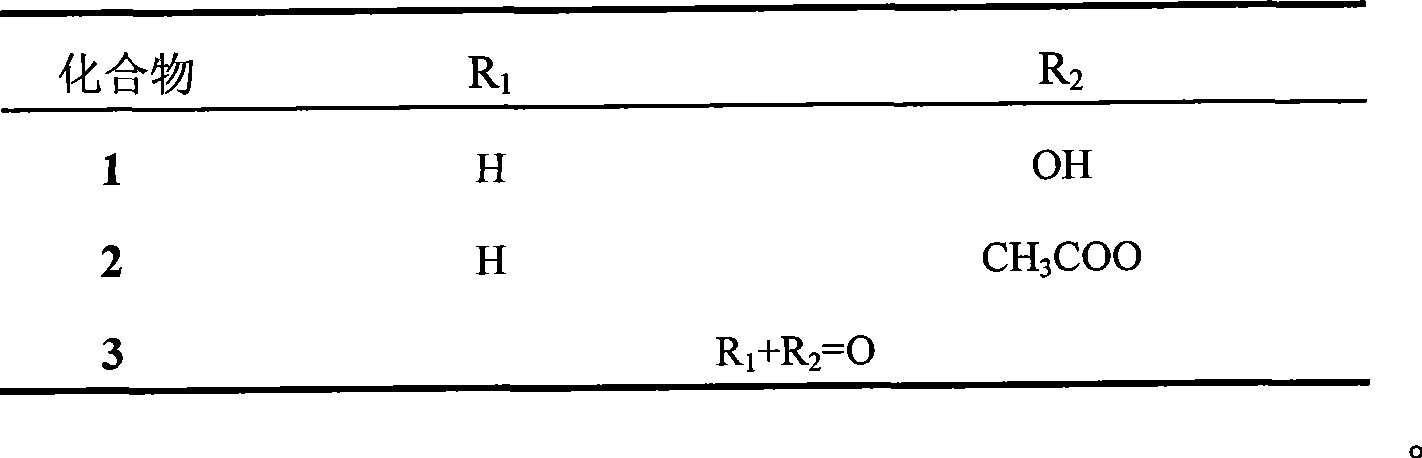
[0023] Heqing schisandra fruit 8kg, soaked in 95% ethanol, extracted with diethyl ether after the extract was concentrated, totally obtained 400 grams (extraction rate 5%), got 200 grams dry loading and carried out silica gel column chromatography, washed with petroleum ether-acetone gradient take off. Petroleum ether-acetone (20:1) fraction, the pigment was removed by MCI column (methanol-water (4:1)), eluted by petroleum ether-acetone (20:1) repeatedly on silica gel column, and then prepared by TLC method (petroleum ether-ethyl acetate (10:1)), to obtain Heqing zizi ethylene (2); the flow fraction of petroleum ether-acetone (10:1), through the MCI column (methanol-water (4:1) ) to remove the pigment, and repeatedly eluted with a silica gel column through petroleum ether-acetone (10:1), and then through the preparative TLC method (petroleum ether-ethyl acetate (6:1)), to obtain Heqing zizi allyl (3); Petroleum ether-a...
Embodiment 2
[0024] Example 2 In vitro anti-HBV experiment
[0025] Apply the 2.2.15 cell line of Hep G2 (Ministry of Education / Ministry of Health Key Laboratory of Medical Molecular Virology, Shanghai), with 10×10 cells per well. 5 Cells were inoculated in a 24-well plate, the medium was DMEM, the growth medium contained 10% fetal bovine serum, 380 μg / ml G418, 0.03% glutamine, 100 μg / ml each of penicillin and streptomycin, in 5% CO 2 Cultivate in an incubator at 37°C. After 48 hours, replace it with a drug-containing culture solution aided by dimethyl sulfoxide. Set 3 to 5 concentrations for each drug, and set 4 parallel wells for each concentration, and continue to cultivate for 9 days. (Change the medium once every 3 days), collect the supernatant and detect the content of HBsAg and HBeAg by ELISA. Under the same conditions, the culture supernatant without drugs was used as the control group. At the same time, the above cell lines were used to measure the cytotoxicity of the drug by M...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com