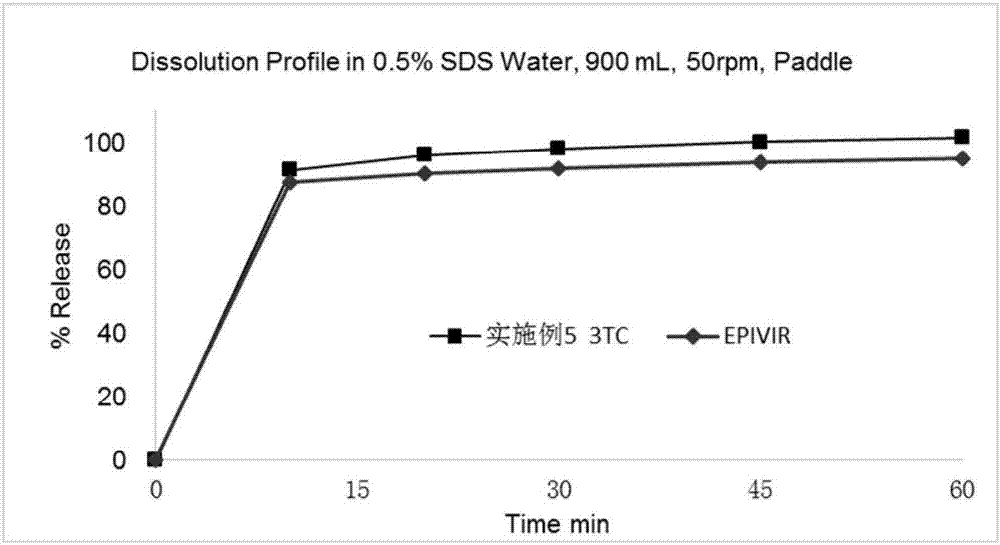
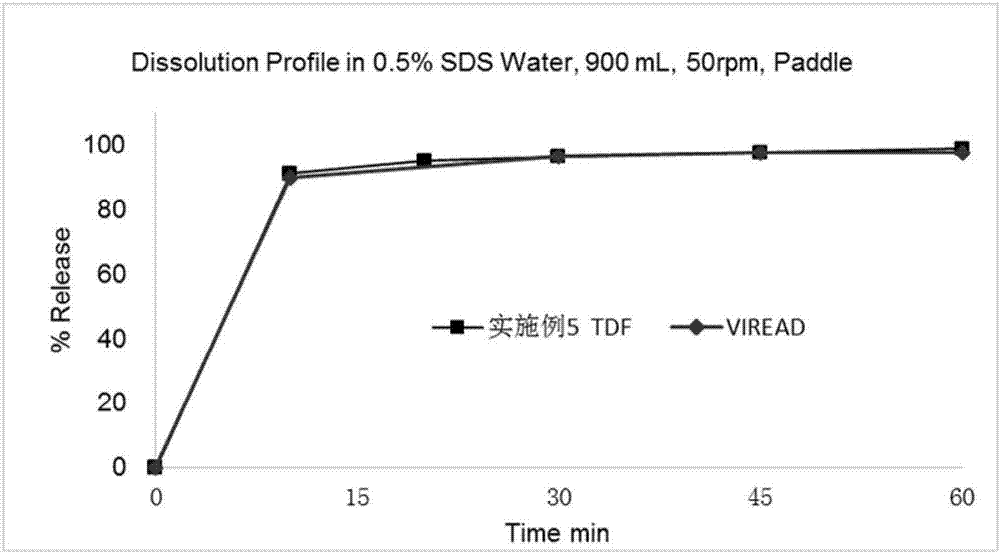
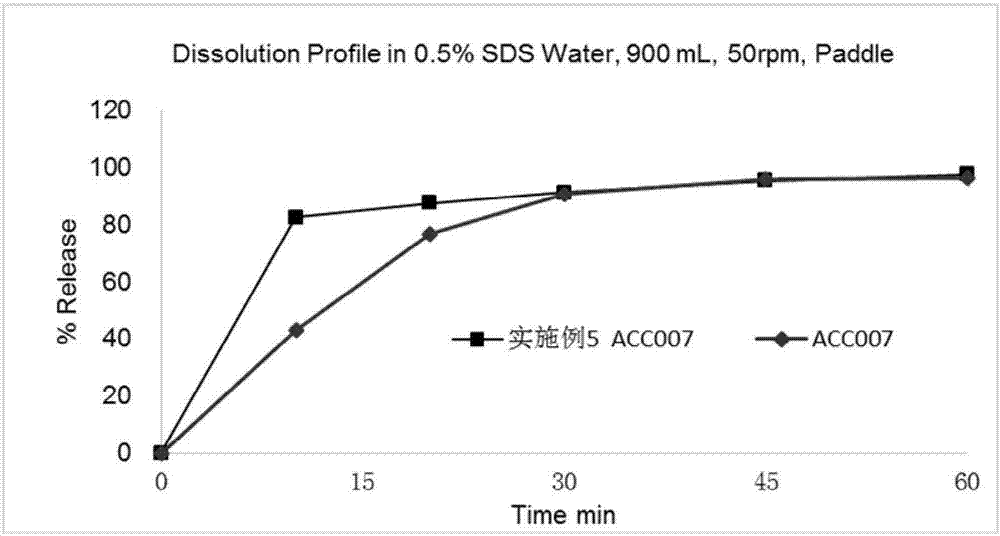
Anti-HIV compound preparation and preparation method and application thereof
A compound preparation and excipient technology, which is applied in the directions of antiviral agents, medical preparations without active ingredients, and medical preparations containing active ingredients, etc. Many problems, to achieve the effect of excellent activity, reducing drug resistance, and a wide range of safe doses
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0052] Table 1 Example 1 prescription component table
[0053]
[0054] The preparation steps are as follows:
[0055] (1) Preparation of tenofovir disoproxil fumarate (TDF) granules: weigh tenofovir disoproxil fumarate and excipient II according to the above formula ratio, pass through a 20-mesh sieve and mix evenly, add the above raw and auxiliary materials 20-60% of the total weight of water is used for wet granulation, and then air blast or fluidized bed drying is used until the moisture content of the granules is less than 5%, and the granules are passed through a 10-mesh sieve to obtain tenofovir disoproxil fumarate granules;
[0056] (2) Preparation of ACC007 granules: Weigh ACC007 and excipient III according to the above formula ratio, pass through a 20-mesh sieve and mix evenly, add 20-60% of the total weight of the above raw and auxiliary materials for wet granulation, and then use air blast Or fluidized bed drying until the moisture content of the granules is le...
Embodiment 2
[0061] Table 2 embodiment 2 prescription component list
[0062]
[0063] The preparation steps are as follows:
[0064] (1) Preparation of tenofovir disoproxil fumarate (TDF) granules: weigh tenofovir disoproxil fumarate and excipient II according to the above formula ratio, pass through a 100-mesh sieve and mix evenly, add the above raw and auxiliary materials 20-60% of the total weight of water is used for wet granulation, and then air blast or fluidized bed drying is used until the moisture content of the granules is less than 4%, and the granules are passed through a 40-mesh sieve to obtain tenofovir disoproxil fumarate granules;
[0065] (2) Preparation of ACC007 granules: Weigh ACC007 and excipient III according to the above formula ratio, pass through a 100-mesh sieve and mix evenly, add 20-60% of the total weight of the above raw and auxiliary materials for wet granulation, and then use air blast Or fluidized bed drying until the moisture content of the granules i...
Embodiment 3
[0069] Table 3 Example 3 prescription component table
[0070]
[0071] The preparation steps are as follows:
[0072] (1) Preparation of tenofovir disoproxil fumarate (TDF) granules: weigh tenofovir disoproxil fumarate and excipient II according to the above formula ratio, pass through a 50-mesh sieve and mix evenly, add the above raw and auxiliary materials 20-60% of the total weight of water is used for wet granulation, and then air blast or fluidized bed drying is used until the moisture content of the granules is less than 3%, and the granules are passed through a 20-mesh sieve to obtain tenofovir disoproxil fumarate granules;
[0073] (2) Preparation of ACC007 granules: Weigh ACC007 and excipient III according to the above formula ratio, pass through a 50-mesh sieve and mix evenly, add 20-60% of the total weight of the above raw and auxiliary materials for wet granulation, and then use air blast Or fluidized bed drying until the moisture content of the granules is le...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com