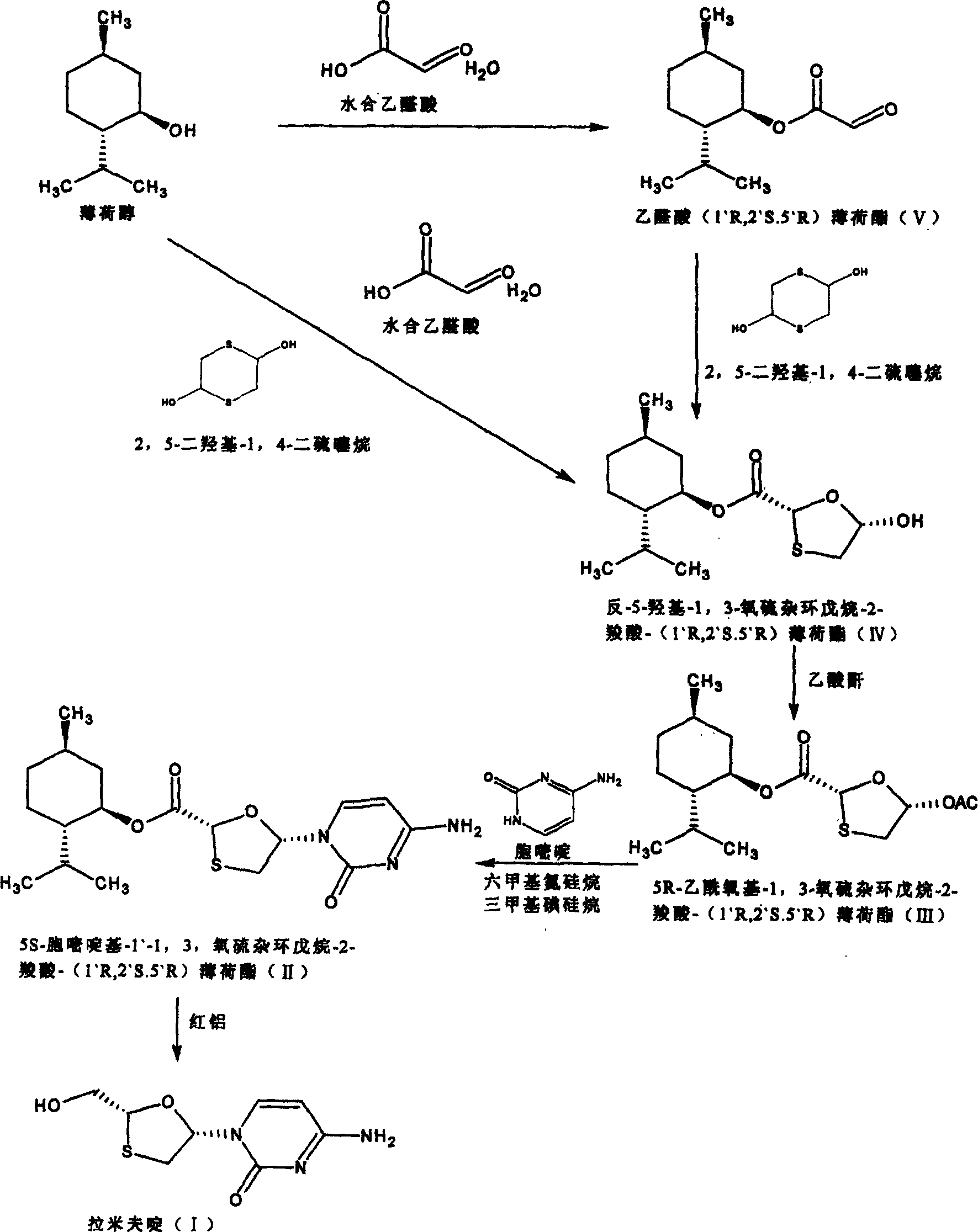
Suitable industrialized method of preparing Lamivudine
A technology of lamivudine and catalyst, applied in the field of preparation of lamivudine, can solve the problems of being unsuitable for large-scale production, time-consuming, low total yield and the like, and achieves easy large-scale production and safety. High, high-yield effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
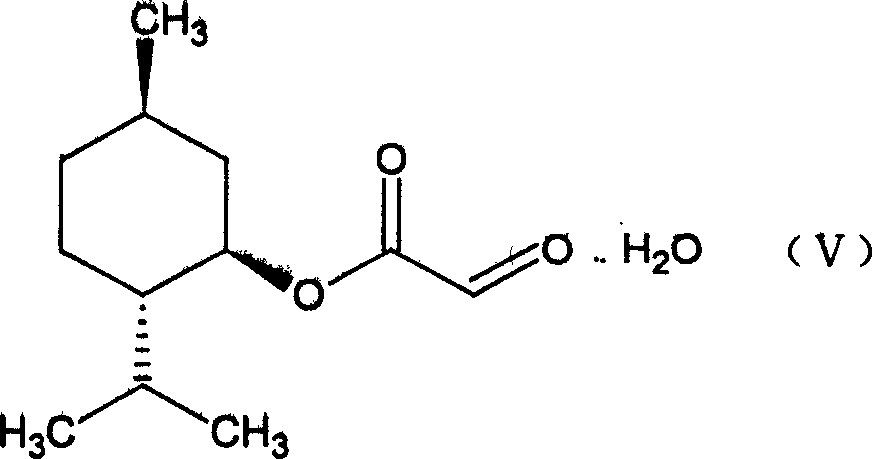
[0022] (1) Preparation of glyoxylic acid (1`R, 2`S, 5`R)-menthyl ester (V)
[0023]
[0024] In a three-necked round bottom flask equipped with a water separator, put 0.11mol of solid glyoxylic acid at room temperature, add 120ml of methyl ether, 0.1mol of menthol, and 1.5g of p-toluenesulfonic acid, heat and stir to dissolve the solid completely, and heat to reflux for 7- After 8 hours, the reaction solution was cooled to room temperature, filtered, washed with 50ml*3 water, the organic phase was collected, dried with anhydrous sodium sulfate overnight, filtered, the solvent was removed under low pressure, the remaining solid was dissolved with a small amount of petroleum ether, and a white solid was obtained 22.4g, melting range: 77-82°C yield: 90%.
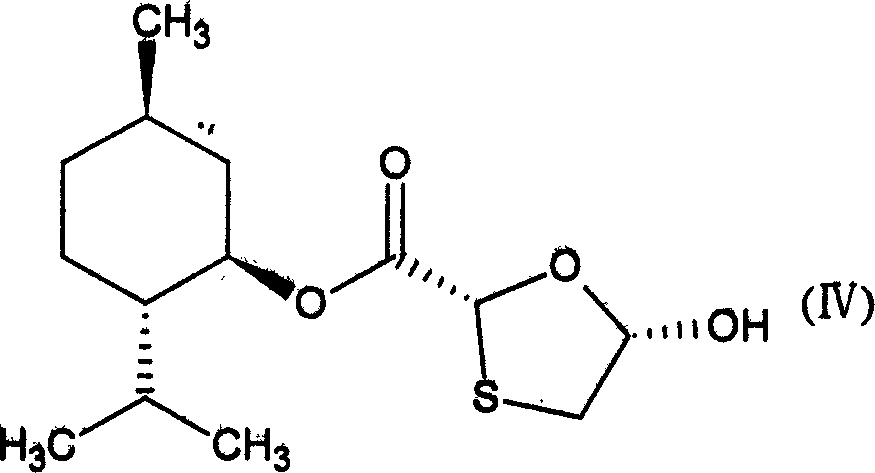
[0025] (2) Preparation of trans-5-hydroxyl-1,3-oxathiolane-2 carboxylic acid-(1`R, 2`S, 5`R)-menthyl ester (IV)
[0026]
[0027] Put 0.1mol glyoxylic acid (1`R, 2`S, 5`R) menthyl ester, 120ml methyl ether, 2,5-dihydroxy...
Embodiment 2
[0038] Preparation of trans-5-hydroxy-1,3-oxathiolane-2-carboxylic acid-(1`R,2`S,5`R)-menthyl ester (IV)
[0039]
[0040] In a three-necked round bottom flask equipped with a water separator, put 0.11mol of solid glyoxylic acid at room temperature, add 120ml of methyl ether, 0.1mol of menthol, and 1.5g of p-toluenesulfonic acid, heat and stir to dissolve the solid completely, and heat to reflux for 7- After 8 hours, cool the reaction solution to room temperature, filter, wash with 50ml*3 water, collect the organic phase, transfer it to a three-necked circular flask, add 0.05mol of 5-dihydroxy-1,4-dithiothiane, heat and stir to 40 ℃, until the white solid is completely dissolved, heat and reflux for 5-6 hours, cool the reaction solution to room temperature, filter, remove the solvent under low pressure, dissolve the remaining white solid with a small amount of petroleum ether, and freeze to obtain an odorous white solid. Yield 45 %, melting range: 110-112°C.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com