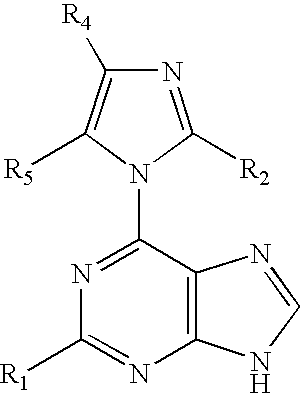
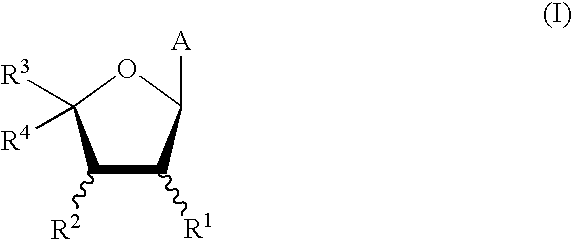
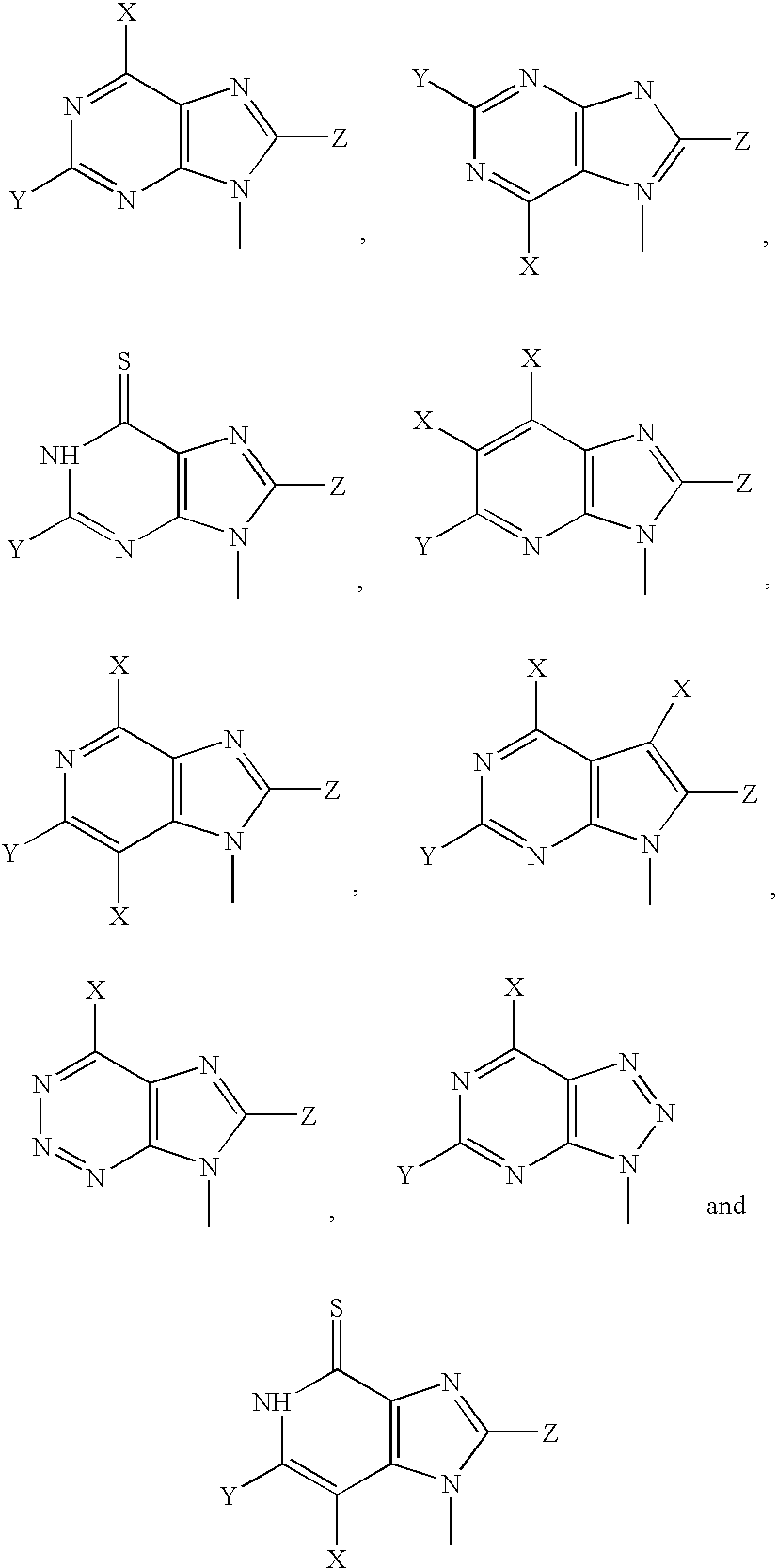
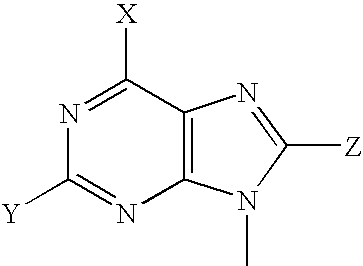
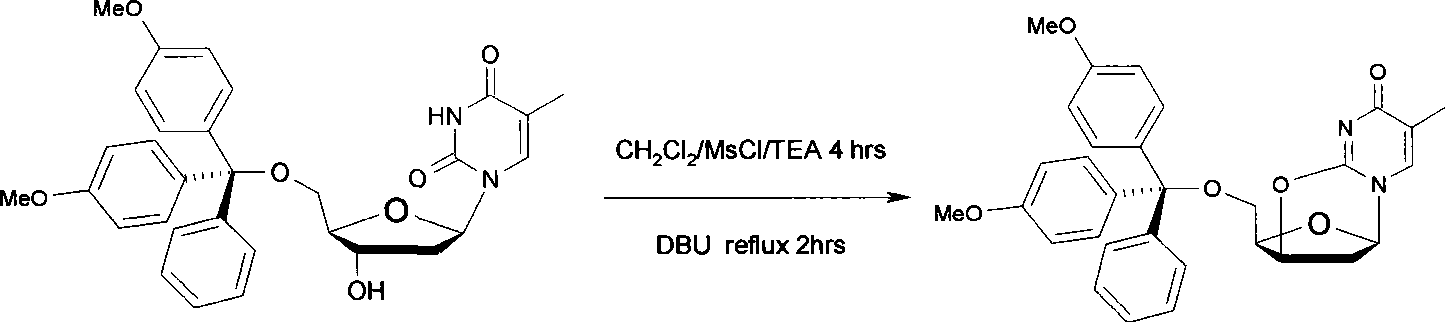
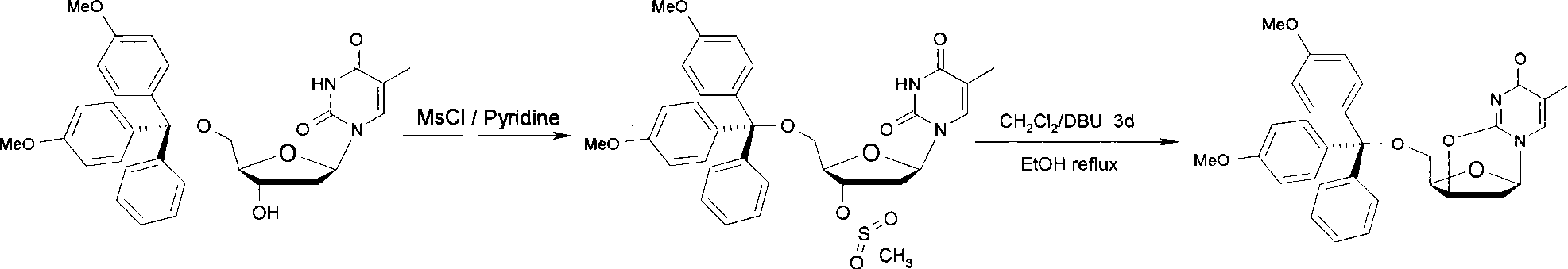
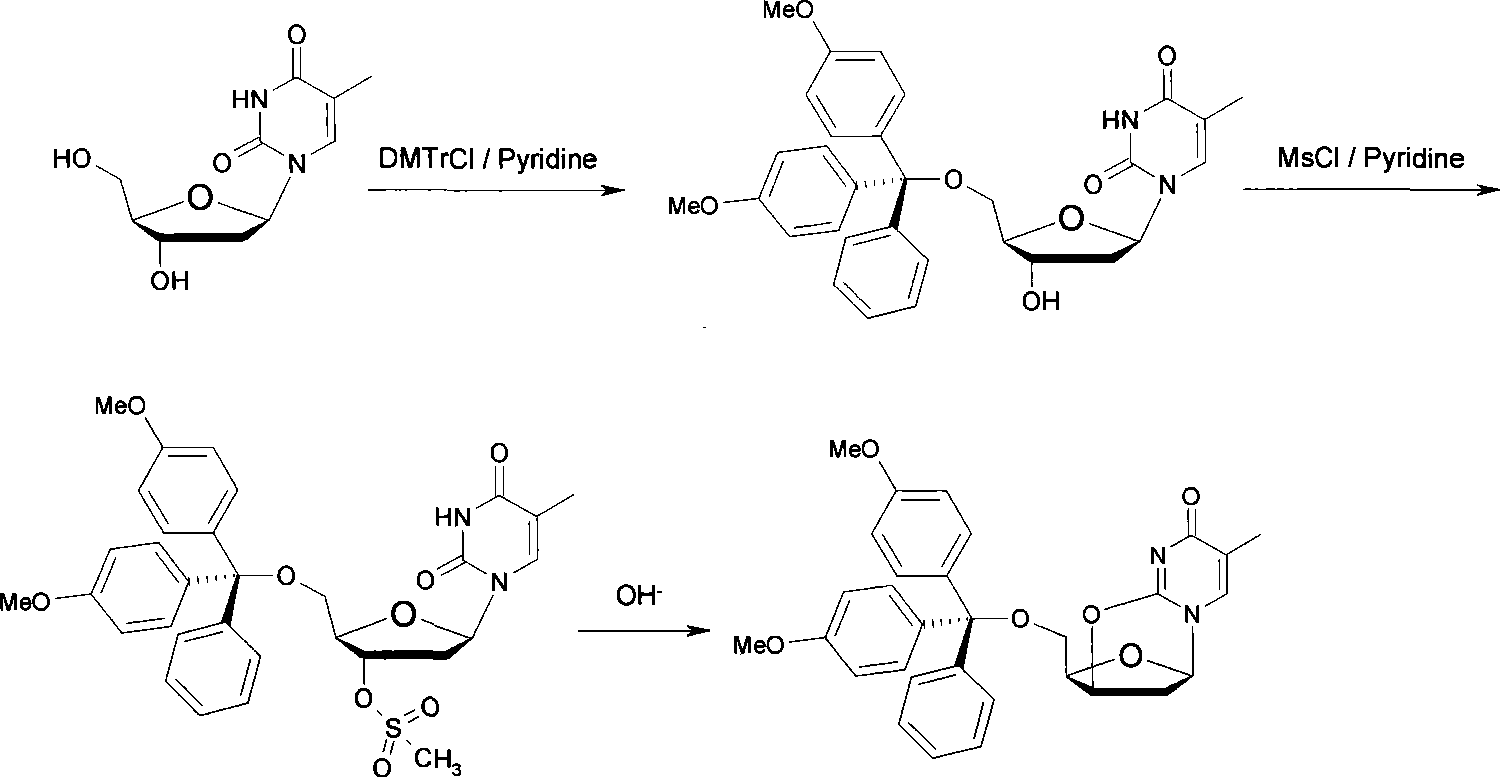
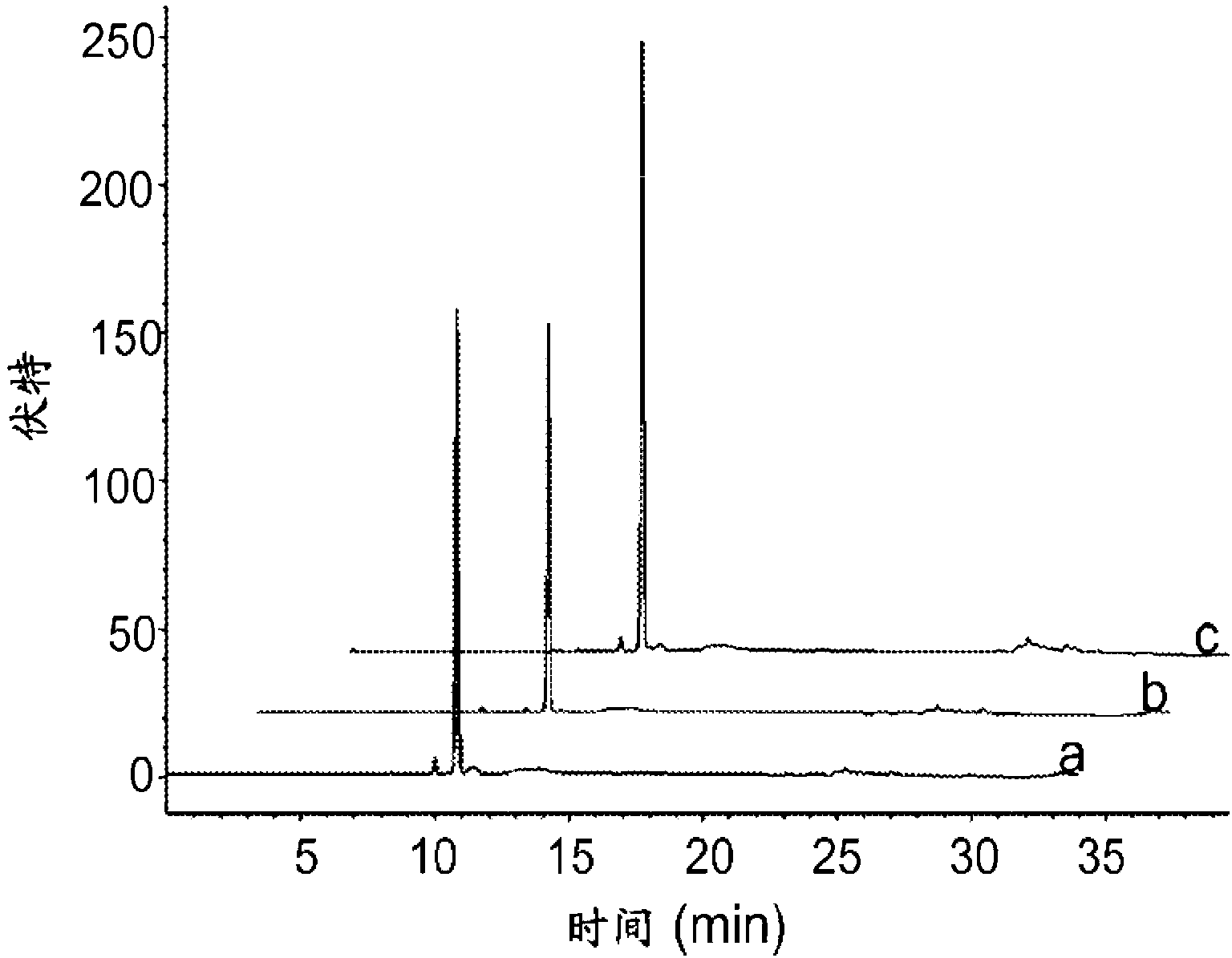
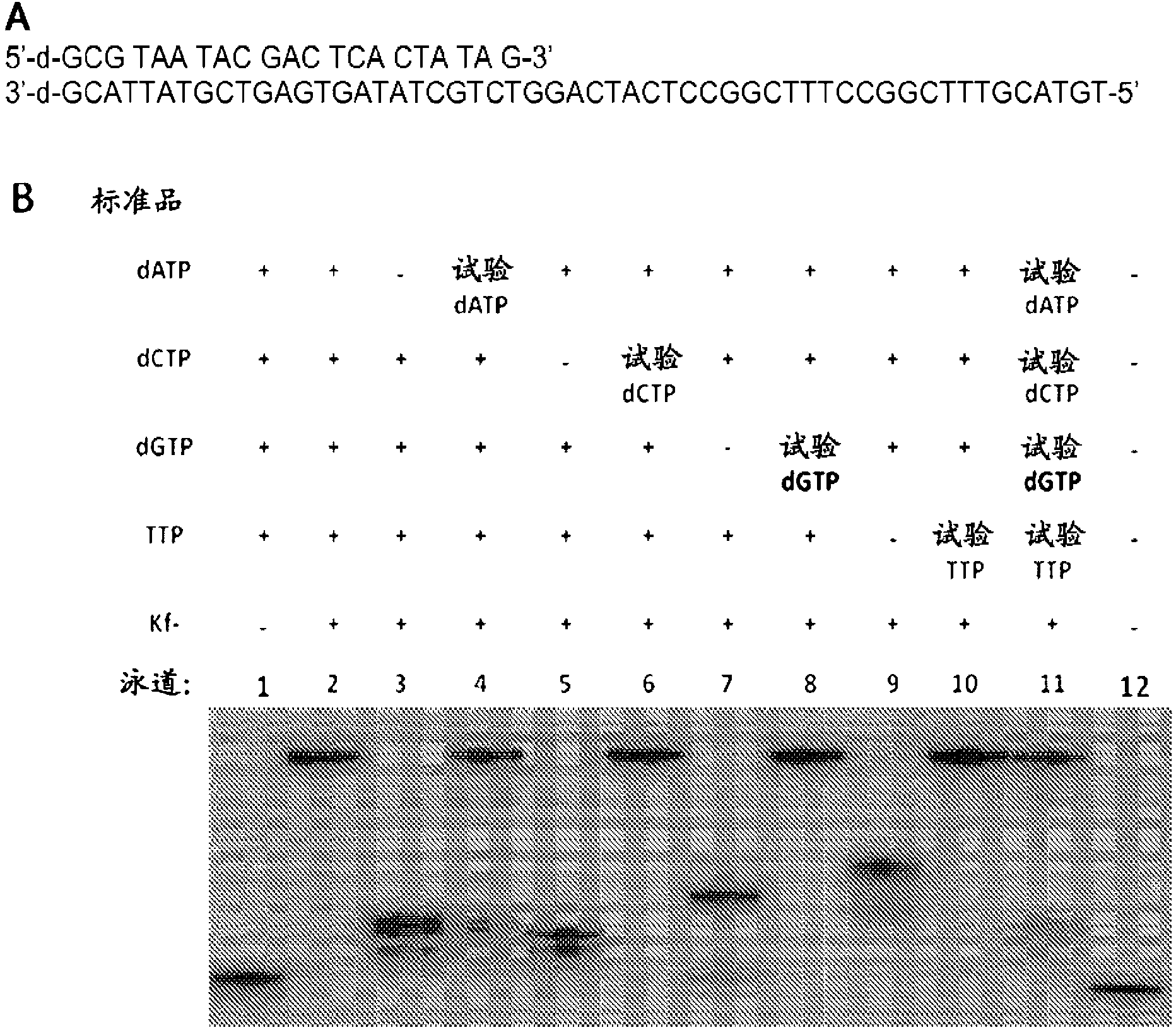
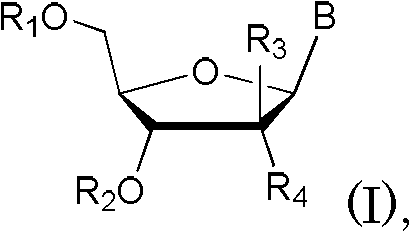
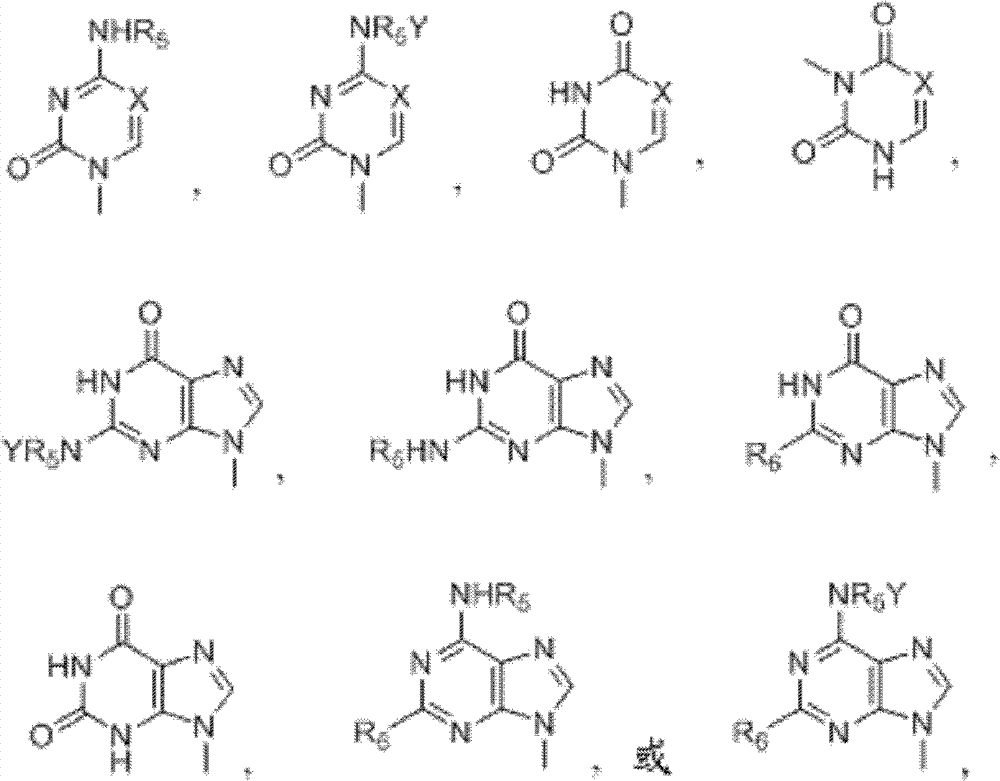
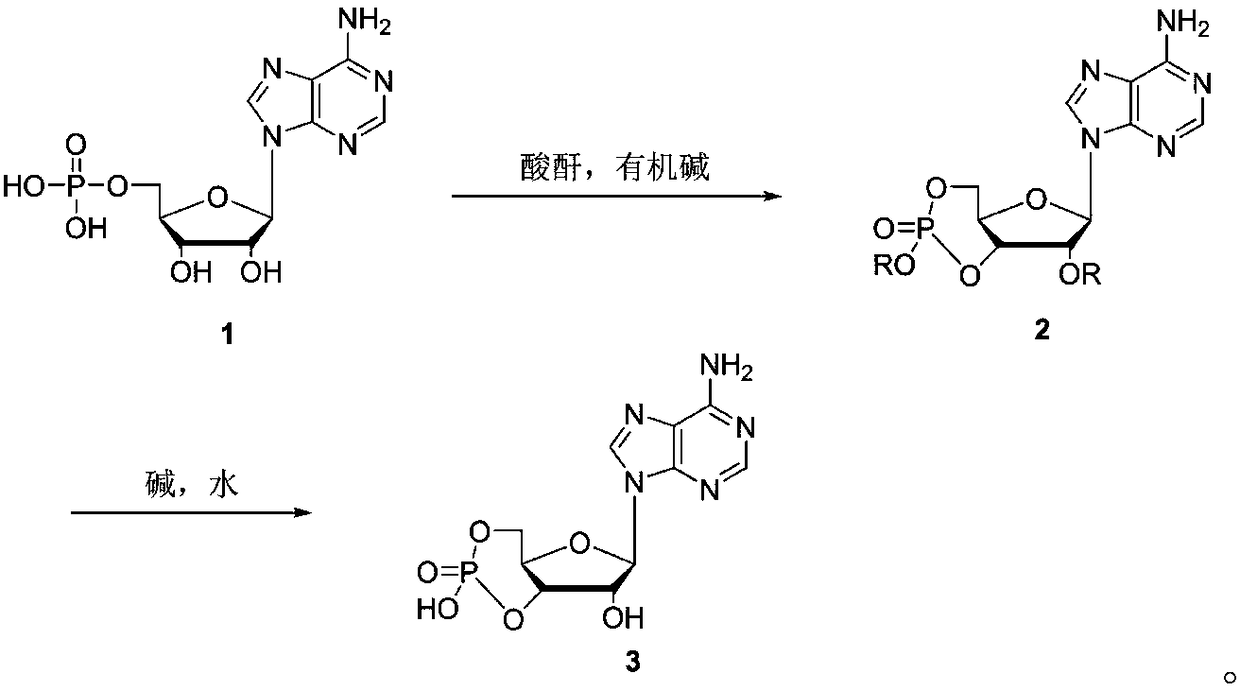
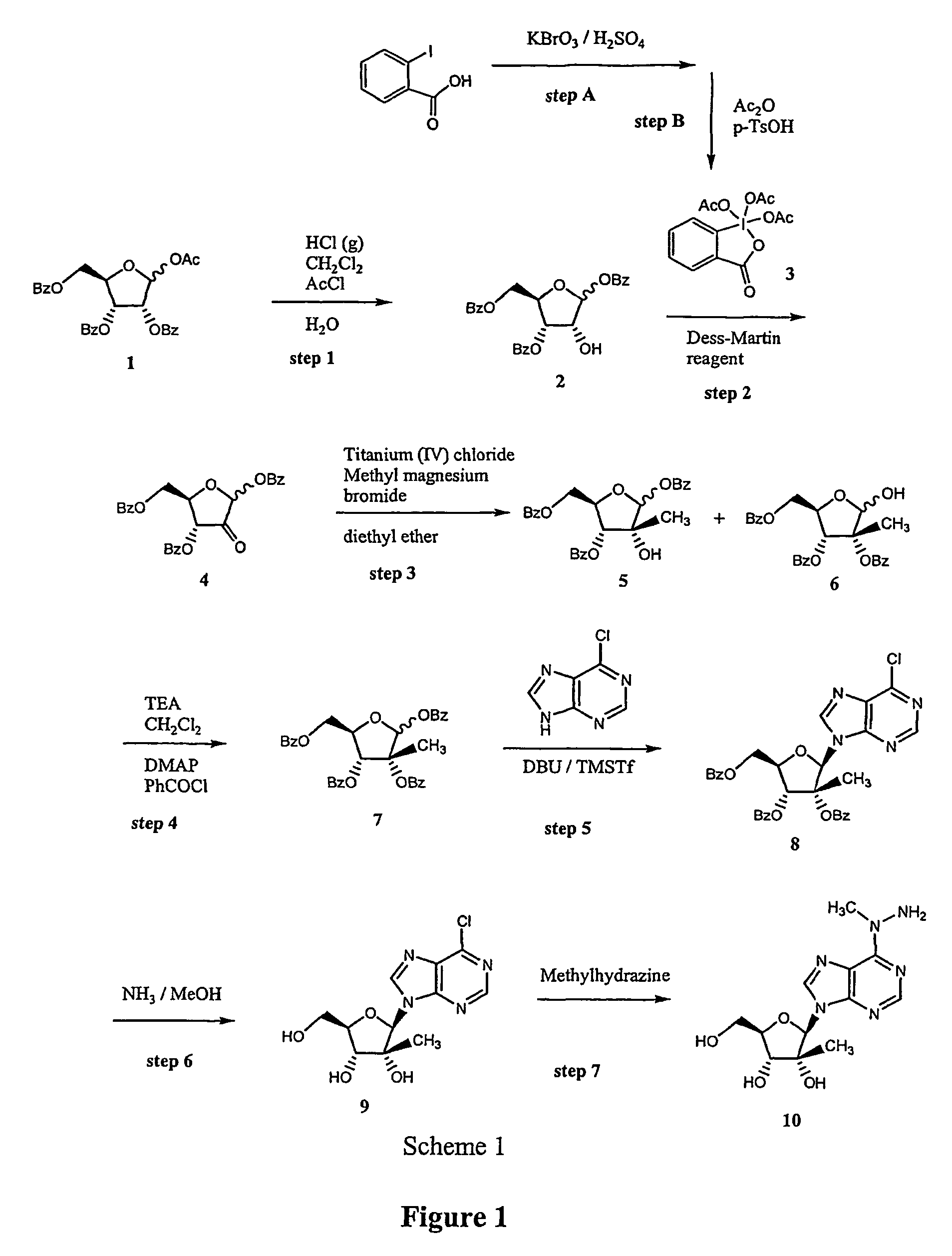
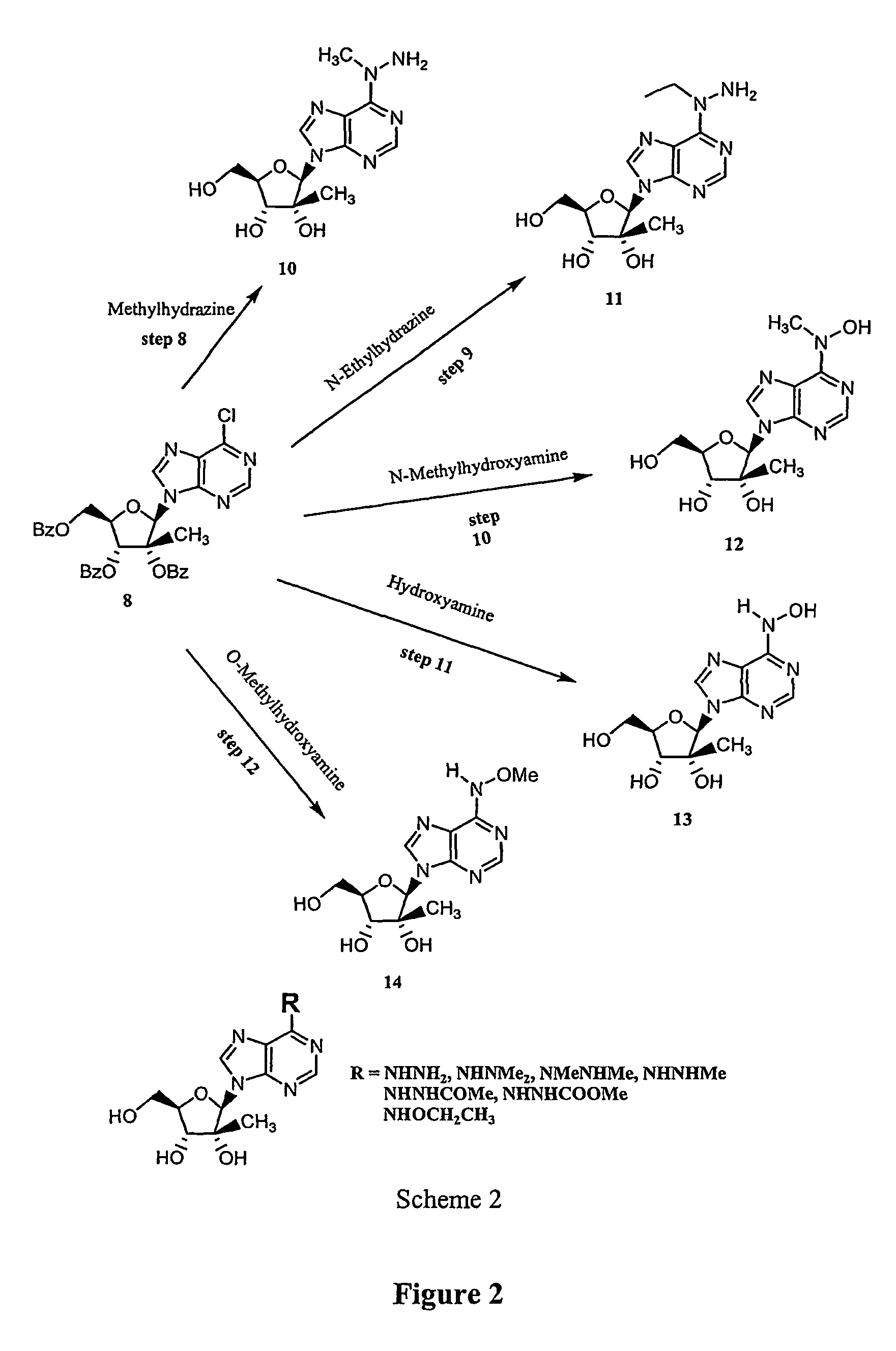
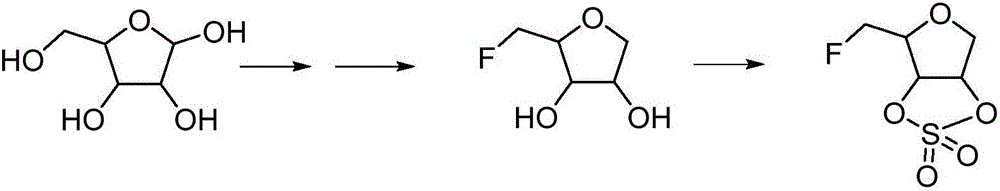
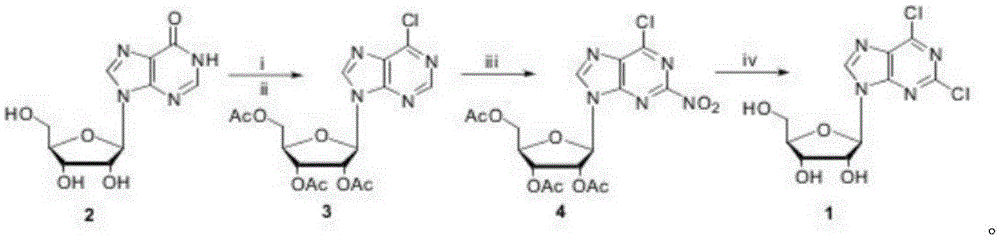
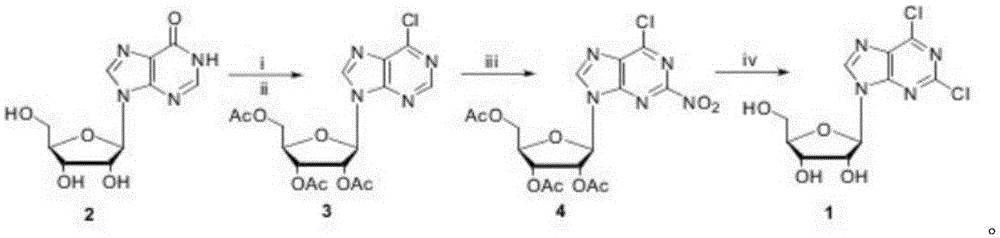
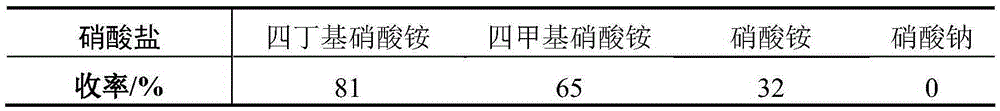
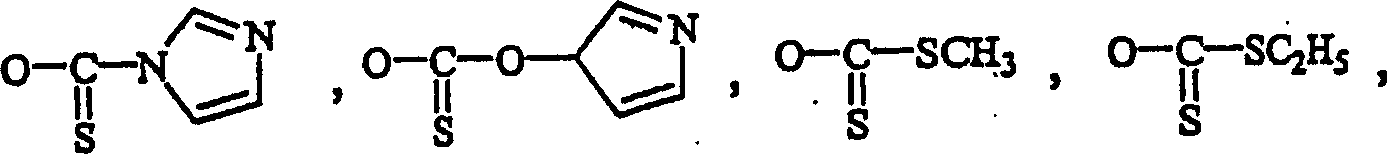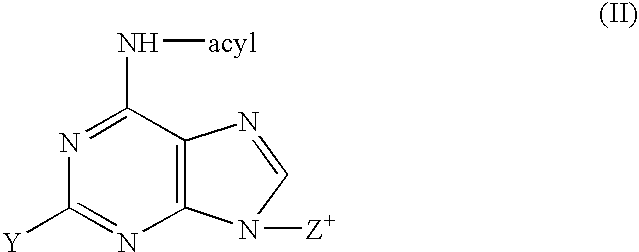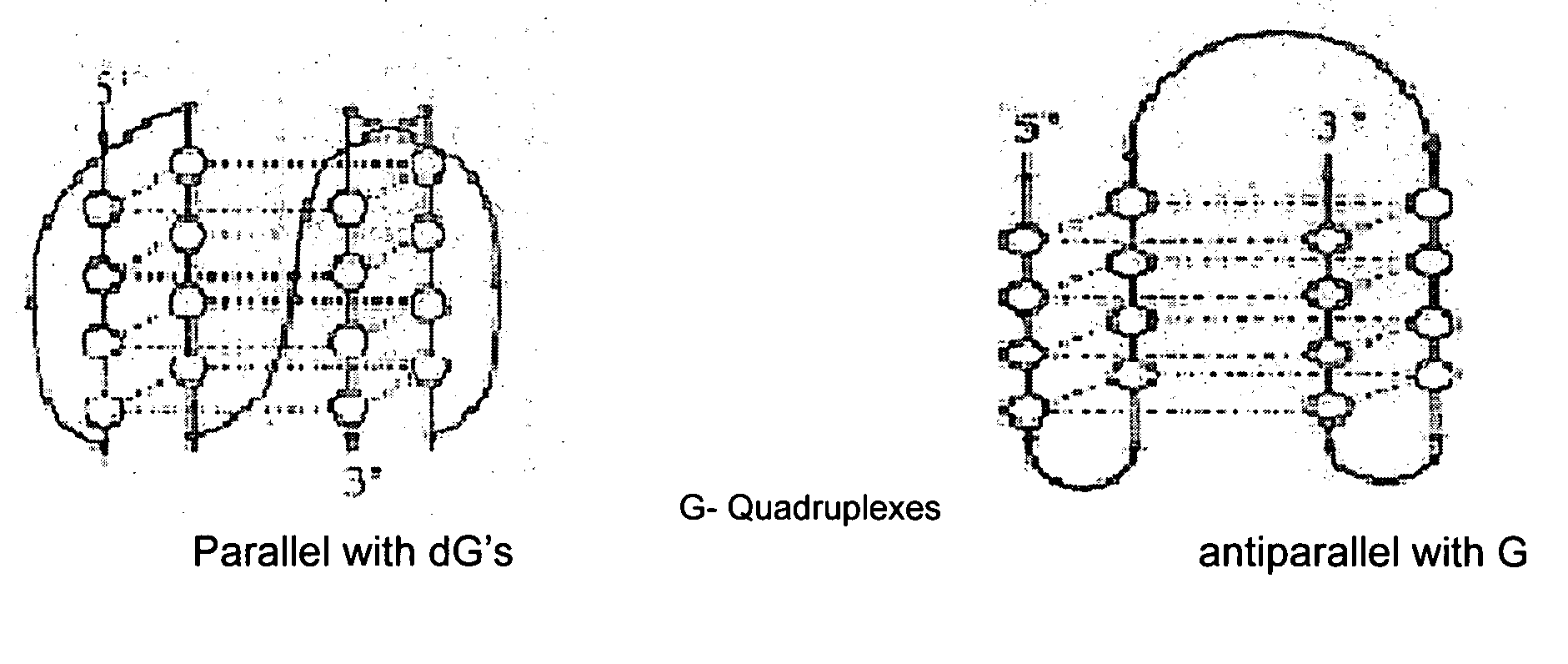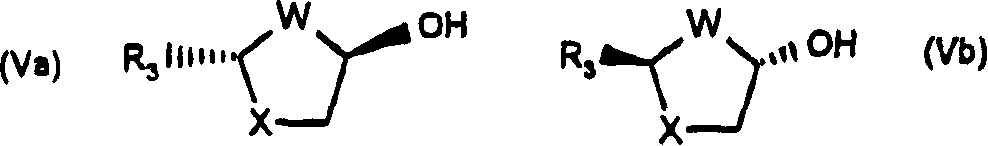Patents
Literature
39 results about "Synthesis of nucleosides" patented technology
Efficacy Topic
Property
Owner
Technical Advancement
Application Domain
Technology Topic
Technology Field Word
Patent Country/Region
Patent Type
Patent Status
Application Year
Inventor
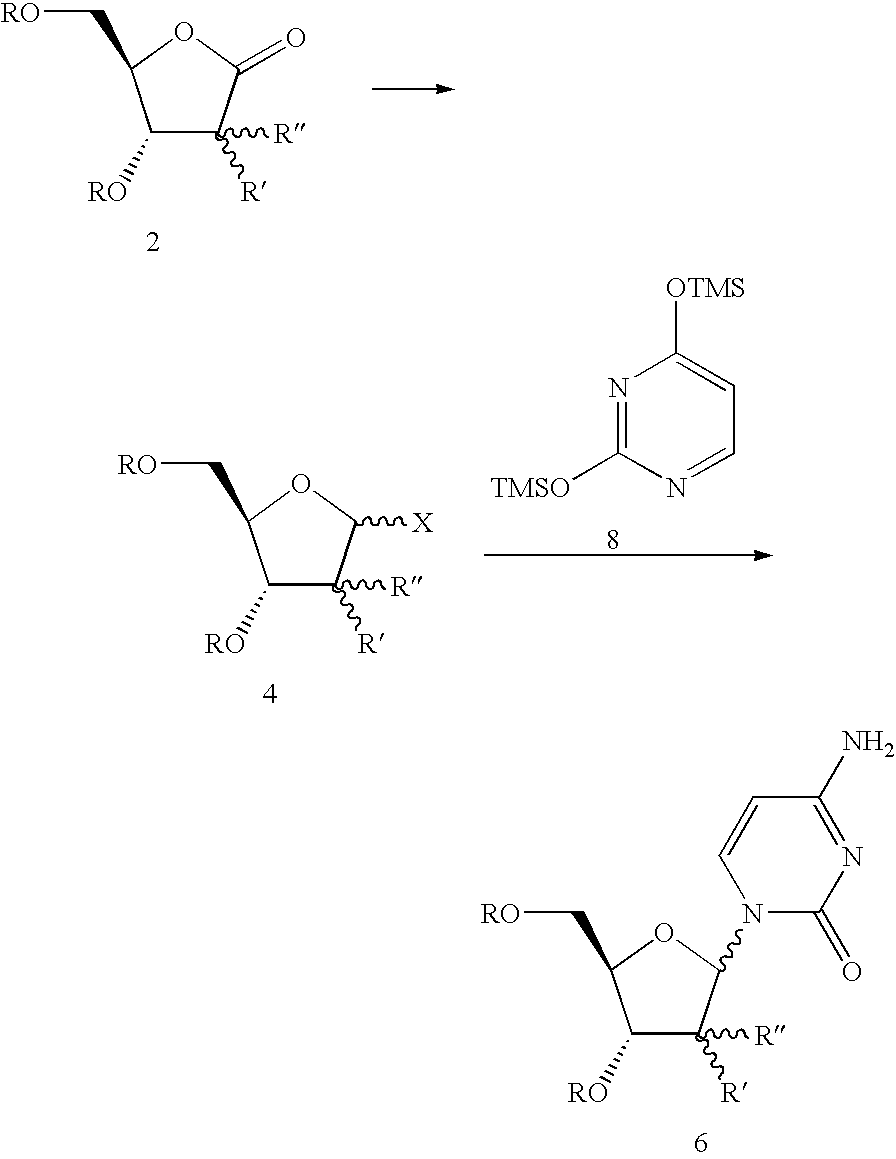
Synthesis of nucleosides involves the coupling of a nucleophilic, heterocyclic base with an electrophilic sugar. The silyl-Hilbert-Johnson (or Vorbrüggen) reaction, which employs silylated heterocyclic bases and electrophilic sugar derivatives in the presence of a Lewis acid, is the most common method for forming nucleosides in this manner.
Preparation of nucleosides ribofuranosyl pyrimidines
InactiveUS20080139802A1Efficient scalable processEasy to handleEsterified saccharide compoundsSugar derivativesCombinatorial chemistrySynthesis of nucleosides
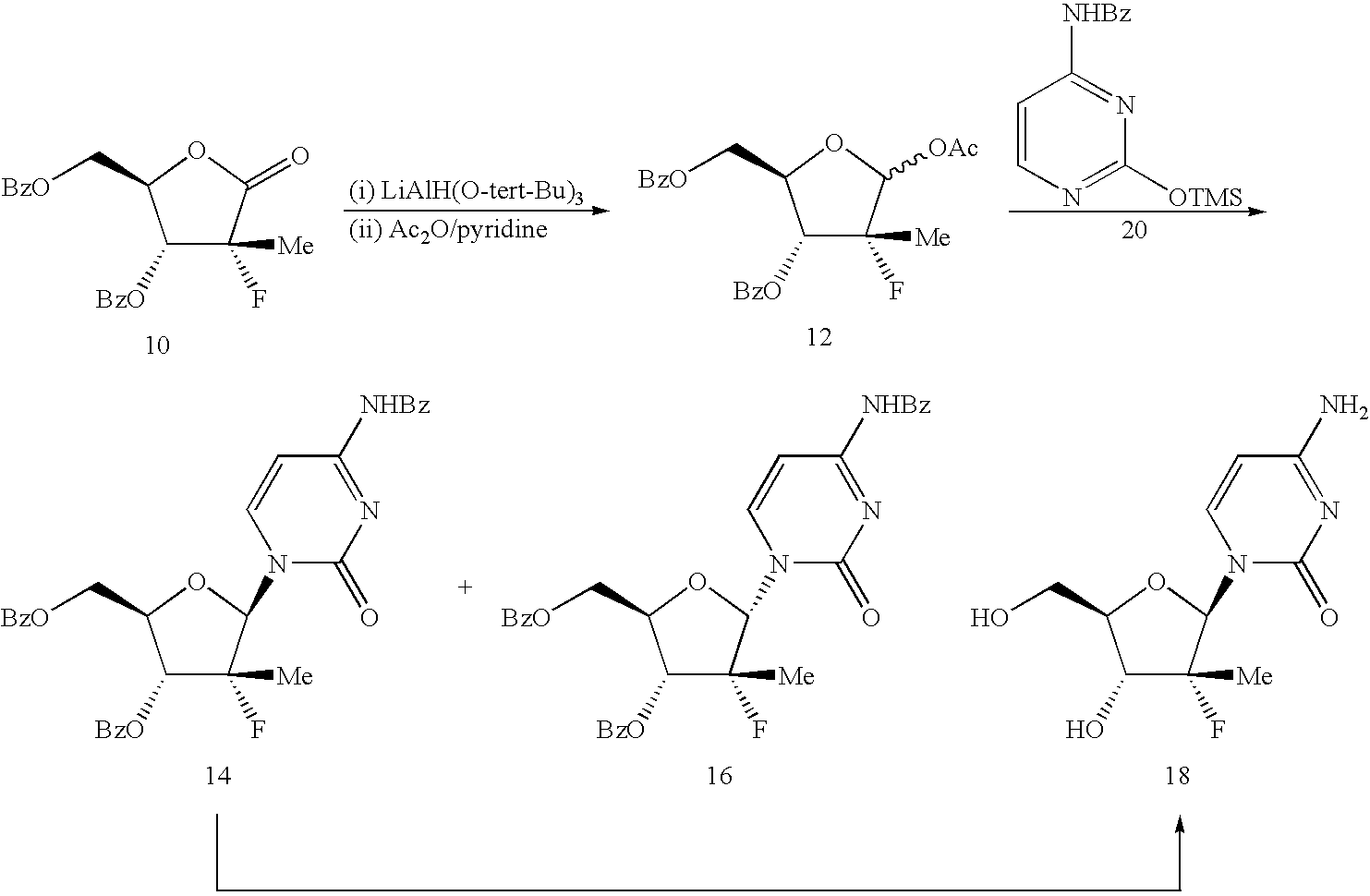
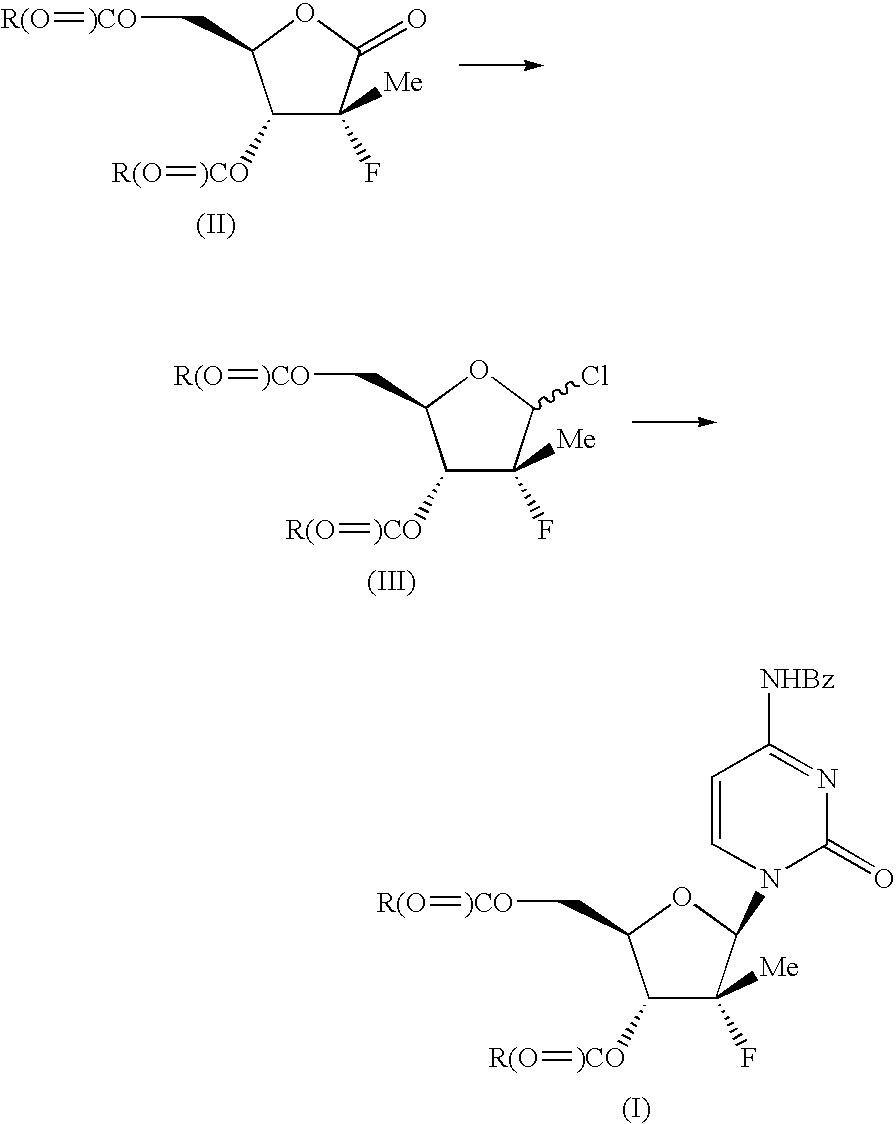
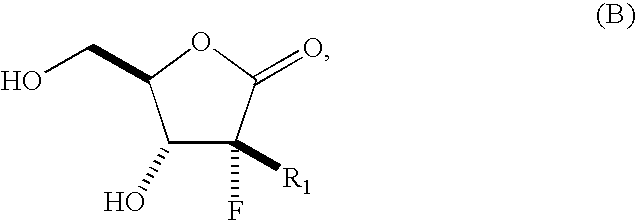
The present process provides an improved method for converting 2′-deoxy-2′-fluoro-2′-methyl-D-ribonolactones derivatives to 3-fluoro-3-methyl-2-chlorofuran compounds which are useful for the synthesis of nucleosides and improved processes for the synthesis of the D-ribonolactone compounds.
Owner:PHARMASSET +1
Processes and intermediates for the preparation of 1'-substituted carba-nucleoside analogs
ActiveUS20110230654A1Saccharide with heterocyclic radicalsOrganic active ingredientsCombinatorial chemistrySynthesis of nucleosides
Owner:GILEAD SCI INC
Process for preparing a synthetic intermediate for preparation of branched nucleosides
ActiveUS7781576B2High yieldSugar derivativesAminosugarsCombinatorial chemistrySynthesis of nucleosides
Owner:INDENIX PHARM LLC
Mutant purine nucleoside phosphorylase proteins and cellular delivery thereof
A host cell stably transformed or transfected by a vector including a DNA sequence encoding for mutant purine nucleoside cleavage enzymes is provided. The transformed or transfected host cell can be used in combination with a purine substrate to treat tumour cells and / or virally infected cells. A nucleotide sequence encoding mutant E. coli derived purine nucleoside phosphorylase proteins which can be used in conjunction with an appropriate substrate to produce toxins which impair abnormal cell growth is also provided. A method is detailed for the delivery of toxin by generation withing target cells or by administration and delivery to the cells from without. Novel purine nucleosides are detailed that yield a cytotoxic purine upn enzymatic cleavage. A synthetic process for nucleosides is also detailed.
Owner:CORNELL CENT FOR TECH ENTERPRISE & COIMMERCIALIZATION +2
Processes and intermediates for the preparation of 1′-substituted carba-nucleoside analogs
ActiveUS10023600B2Organic active ingredientsSaccharide with heterocyclic radicalsCombinatorial chemistrySynthesis of nucleosides
Owner:GILEAD SCI INC
Synthesis of 2'-deoxy-l-nucleosides
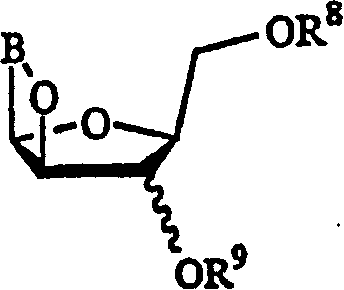
This invention provides processes for the preparation of compounds having the structure: wherein X and Y are same or different, and H, OH, OR, SH, SR, NH2, NHR', or NR'R'' Z is H, F, Cl, Br, I, CN, or NH2. R is hydrogen, halogen, lower alkyl of C1-C6 or aralkyl, NO2, NH2, NHR', NR'R'', OH, OR, SH, SR, CN, CONH2, CSNH2, CO2H, CO2R', CH2CO2H, CH2CO2R', CH-CHR, CH2CH-CHR, or C-CR. R' and R'' are same or different, and lower alkyl of C1-C6. R13 is hydrogen, alkyl, acyl, phosphate (monophosphate, diphosphate, triphosphate, or stabilized phosphate) or silyl; and
Owner:法玛赛特有限公司
Methods For Selective N-9 Glycosylation of Purines
A process for providing regiospecific and highly stereoselective synthesis of 9-β anomeric purine nucleoside analogs is described. The introduction of the sugar moiety on to 6-(azolyl)-substituted purine bases is performed so that highly stereoselective formation of the β anomers of only the 9 position regioisomers of the purine nucleoside analogs (either D or L enantiomers) is obtained. This regiospecific and stereoselective introduction of the sugar moiety allows the synthesis of nucleoside analogs, and in particular 2′-deoxy, 3′-deoxy, 2′-deoxy-2′-halo-arabino and 2′,3′-dideoxy-2′-halo-threo purine nucleoside analogs, in high yields without formation of the 7-positional regioisomers. Processes for providing novel 6-(azolyl)purines for the regiospecific and highly stereoselective synthesis of 9-β anomeric purine nucleoside analogs are described. The compounds are drugs or intermediates to drugs.
Owner:BRIGHAM YOUNG UNIV
Mutant purine nucleoside phosphorylase proteins and cellular delivery thereof
A host cell stably transformed or transfected by a vector including a DNA sequence encoding for mutant purine nucleoside cleavage enzymes is provided. The transformed or transfected host cell can be used in combination with a purine substrate to treat tumor cells and / or virally infected cells. A nucleotide sequence encoding mutant E. coli derived purine nucleoside phosphorylase proteins which can be used in conjunction with an appropriate substrate to produce toxins which impair abnormal cell growth is also provided. A method is detailed for the delivery of toxin by generation within target cells or by administration and delivery to the cells from without. Novel purine nucleosides are detailed that yield a cytotoxic purine upon enzymatic cleavage. A synthetic process for nucleosides is also detailed.
Owner:CORNELL CENT FOR TECH ENTERPRISE & COIMMERCIALIZATION +2
Process for the preparation of 9-beta-anomeric nucleoside analogs
InactiveUS6884880B2High yieldSugar derivativesMicrobiological testing/measurementRegioselectivityPurine
A process for substantially enhancing the regio and stereoselective synthesis of 9-β-anomeric nucleoside analogs is described. The introduction of the sugar moiety onto a 6-substituted purine base was preformed so that only the 9-β-D- or L-purine nucleoside analogs were obtained. This regio and stereoselective introduction of the sugar moiety allows the synthesis of nucleoside analogs and in particular 2′-deoxy, 3′-deoxy, 2′-deoxy-2′-β-fluoro and 2′,3′-dideoxy-2′-β-fluoro purine nucleoside analogs in high yield without virtually any formation of the 7-positional isomers. The compounds are drugs or intermediates to drugs.
Owner:TALMER BANK & TRUST +1
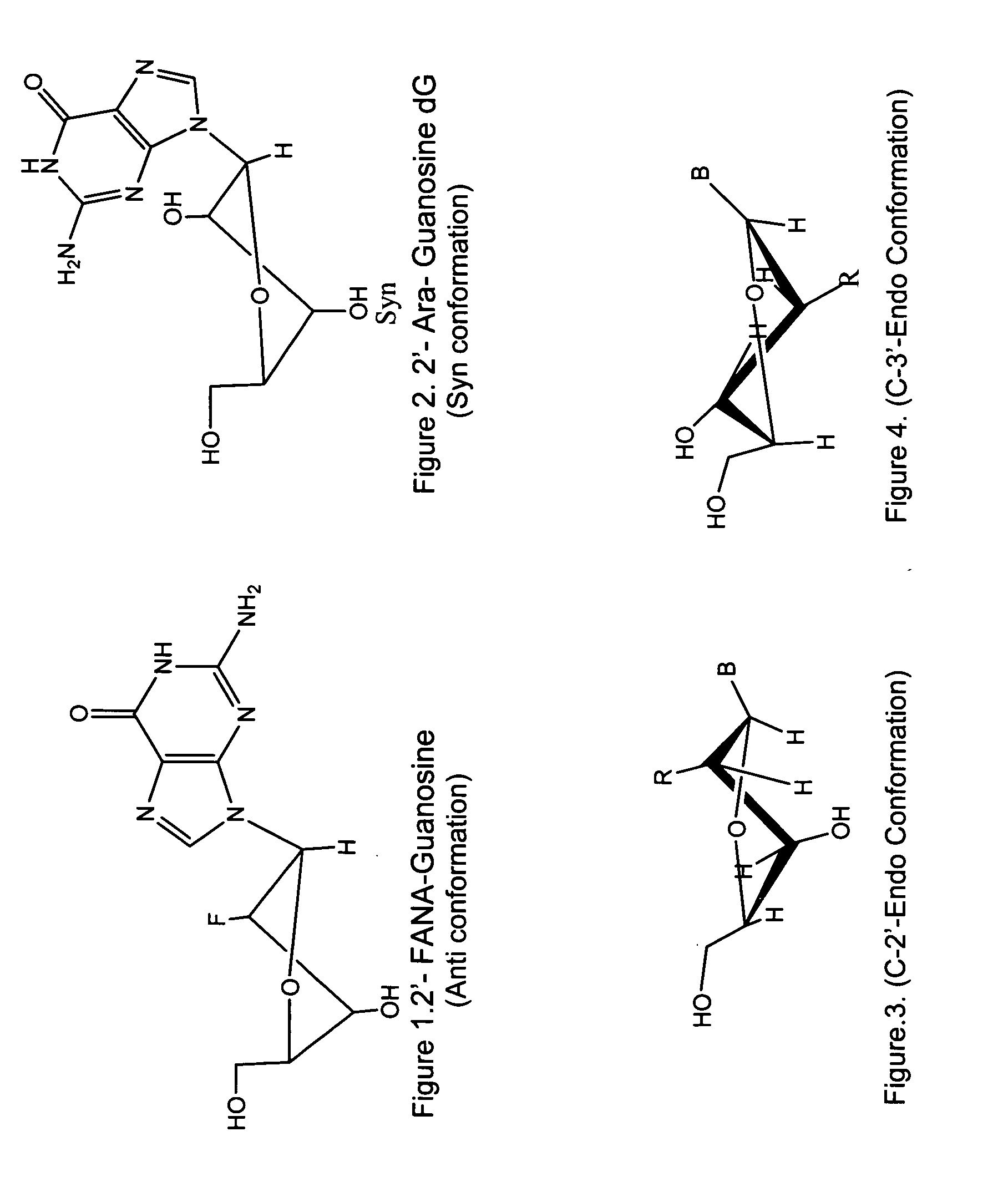
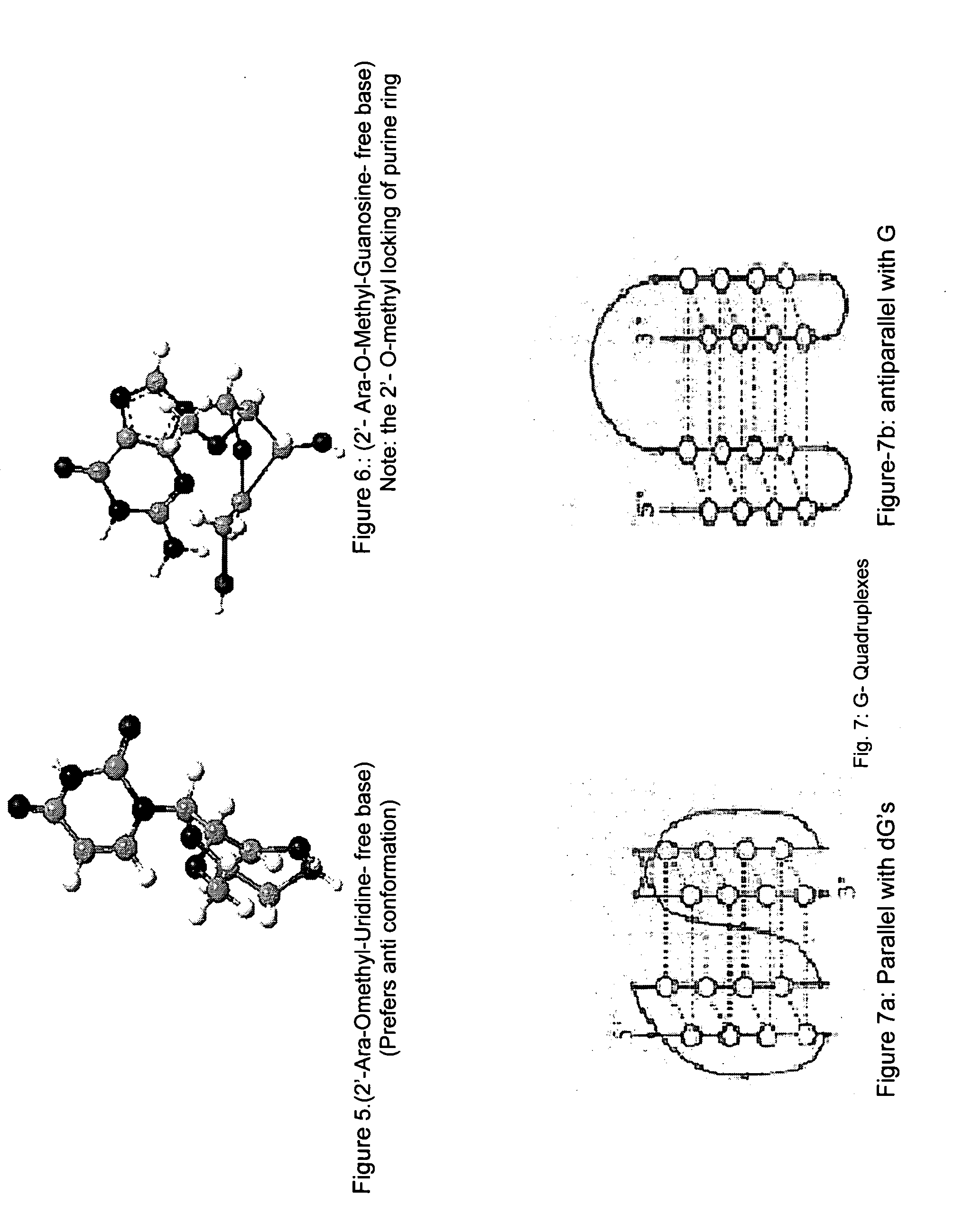
Synthesis of ara-2'-o-methyl-nucleosides, corresponding phosphoramidites and oligonucleotides incorporating novel modifications for biological application in therapeuctics, diagnostics, g- tetrad forming oligonucleotides and aptamers
InactiveUS20120149888A1Prolong lifeEffective interactionOrganic active ingredientsSugar derivativesPhosphateSynthetic DNA
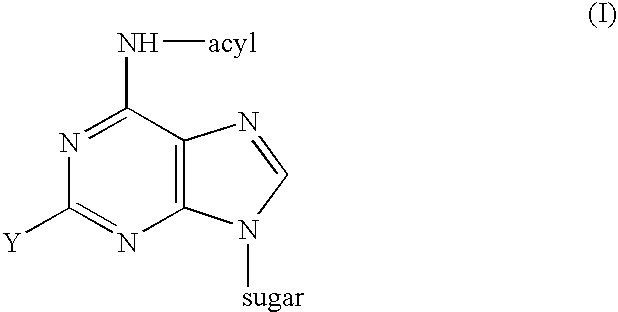
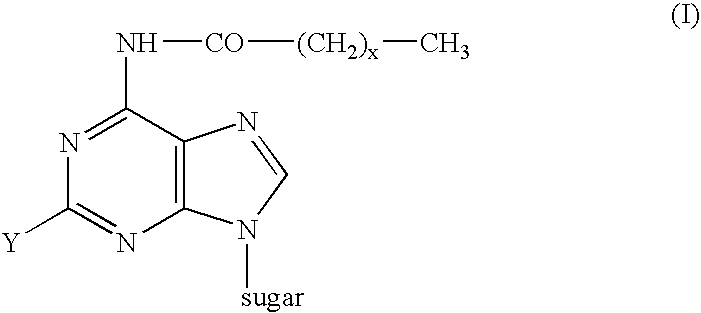
The present invention relates to synthesis, purification and methods to obtain high purity novel 2′-arabino-O-methyl nucleosides and the corresponding phosphoramidites of various arabinonucleoside bases and introduction of such units into defined sequence synthetic DNA and RNA. Various synthetic oligonucleotides, such as HIV integrase inhibitor 14-mer and thrombin binding oligonucleotide, thrombin-1, bearing ara-2′-omethyl modification have been synthesized. It is anticipated the oligonucleotides incorporating these monomers will exhibit biological activities related to antisense approach approach, design of better SiRNA's, diagnostic agents. Similarly, it is anticipated that oligonucleotides incorporating such novel nucleosides will be useful to develop therapeutic candidates designing stable G-quadruplexes and Aptamers for oligonucleotide structure, folding topology, evaluation of biochemical properties and design and develop as therapeutic agents. It is further anticipated that the nucleosides, phosphates and triphosphates of this invention could develop as therapeutic agents.
Owner:CHEMGENES CORP
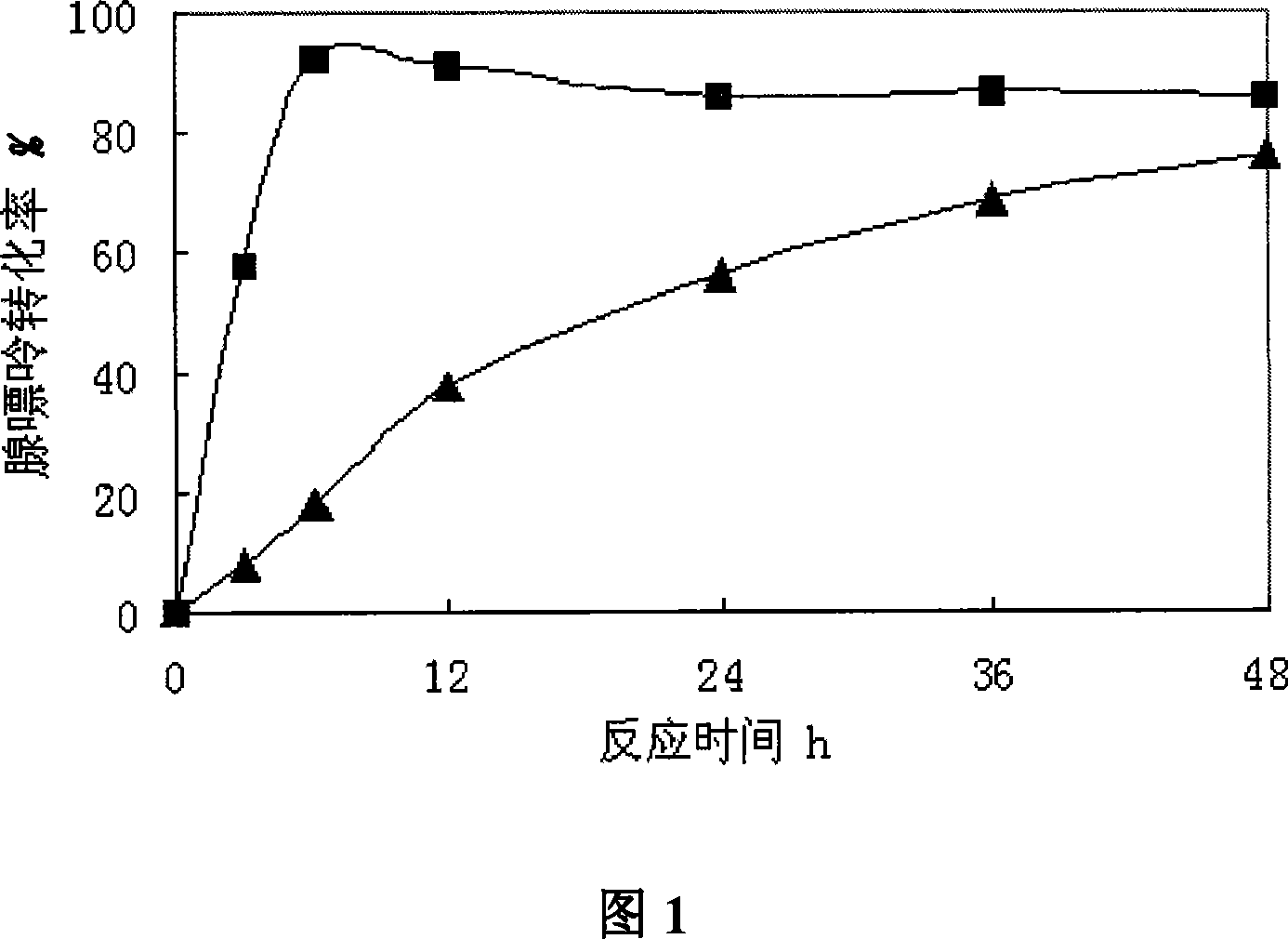
Bacterial with high-yield of nucleoside phosphorylase and method for synthesizing arabinose nucleoside
InactiveCN101113420AIncrease vitalityImprove conversion rateBacteriaFermentationFlucytosineNucleoside phosphorylase
A compound method of high-yield nucleoside phosphorylase strains and arabinose nucleoside pertains to biochemical engineering field, in particular to the high-yield nucleoside phosphorylase strains and a method for compounding arabinose purine nucleoside with the strains by an enzyme method. The invention aims at solving a technical problem for providing the strains of the high-yield nucleoside phosphorylase and strains of uridine phosphorylase and the method for producing the arabinose purine nucleoside with the strains. The invention discloses enterobacter aerogenes with a preservation number of CGMCC No.2035 and the method for producing the arabinose purine nucleoside with the strains, and the invention comprises steps that (1) the enterobacter aerogenes DWOQ-58 of the invention is cultured and collected, and (2) the enterobacter aerogenes DWOQ-58 is contacted with arabinose donor and receptors of purine base. The strains of the invention are rich in vigor and resists 5-flucytosine with an average conversion rate of more than 80 percent in general and the reaction time of the invention is shortened to less than 12 hours.
Owner:SHANGHAI WEIPING BIOLOGICAL TECH
Method for synthesizing positive electron radioactive imaging agent labeled precursor thymidine derivative
InactiveCN101481399ALow costWide variety of sourcesSugar derivativesRadioactive preparation carriersImaging agentChloride
The invention discloses a synthetic method of a labelled precursor of a positron emission developer (a thymidine derivative), in particular relates to a synthetic method of 5'-O-(4,4'-dimethoxytriphenylmethyl)-2,3'-anhydro-thymidine. The synthetic method comprises the following steps: thymidine is taken as a raw material for reacting with 4,4'-dimethoxytriphenylmethyl and methylsulfonyl chloride, an intermediate product is subject to a cyclization reaction in alkaline aqueous solution, and finally separated and purified to obtain a final product. The synthetic method has the advantages of low-cost raw materials, simple operation, few process steps, short reaction time and high product yield.
Owner:GUANGZHOU GENERAL HOSPITAL OF GUANGZHOU MILITARY COMMAND
Novel synthesis of nucleoside 5'-triphosphates and their derivatives
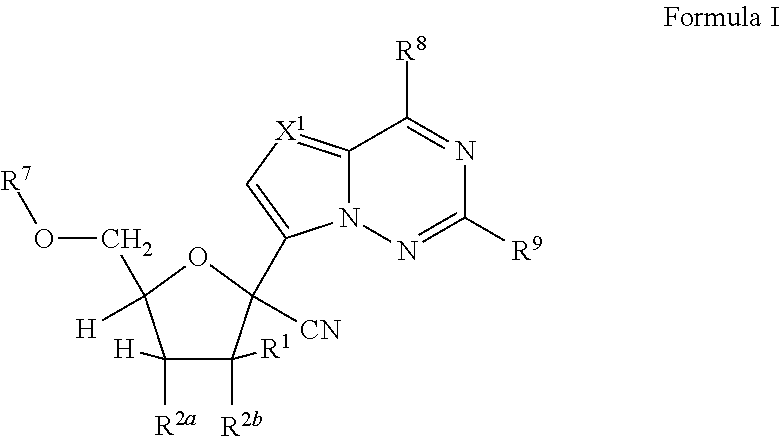
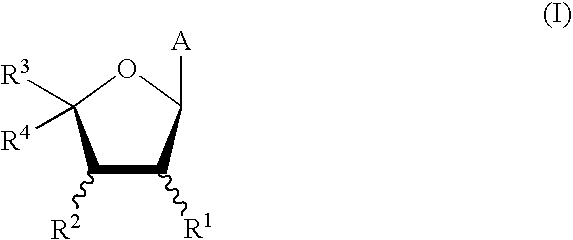
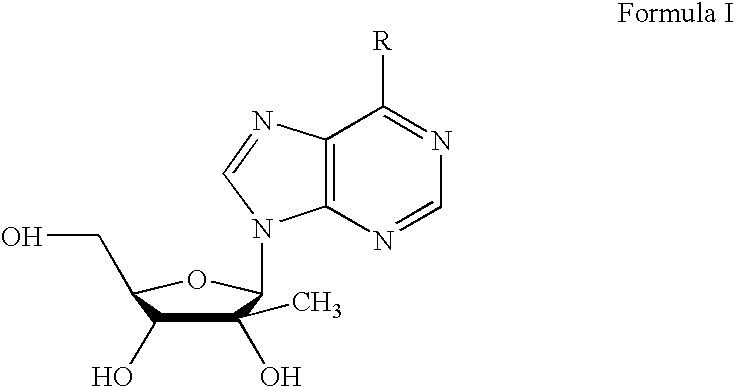
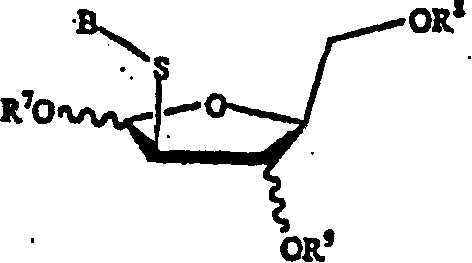
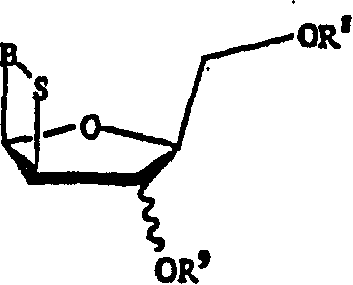
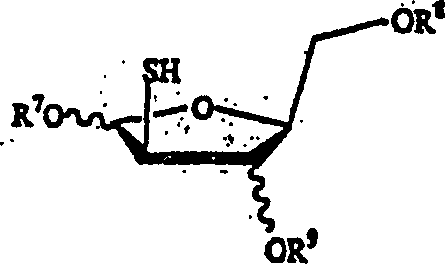
Disclosed are compounds of nucleoside 5 '-triphosphates of formula (I), or derivatives thereof, or pharmaceutically acceptable salts of said nucleoside 5 '-triphosphates or said derivatives, wherein the Base of formula (I) is Adenine (A), Cytosine (C), Guanine (G), Thymine (T), Uracil (U), modified nucleobase or unnatural nucleobase; R1 is H or OH, R2 is H or OH, X is independently selected from the group consisting of O, S and Se; and Y is independently selected from the group consisting of O, B (borano, or BH3), S, and Se. Also disclosed are processes of preparing the compounds of formula (I), said process comprising steps according to Scheme A.
Owner:SENA RES
Stereoselective synthesis of beta-nucleosides
A method of stereoselectively synthesizing a [beta]-nucleoside, e.g., 2'-deoxy-2,2'-difluorocytidine, is described. The method includes reacting a tetrahydrofuran compound of the following formula, wherein R1, R2, R3, R4 and L are defined as in the specification, with a nucleobase derivative in the presence of an oxidizing agent.
Owner:PHARMAESSENTIA CORP
Method for synthesizing 5'-chloro-nucleoside
InactiveCN101250210APromote application developmentMild reaction conditionsSugar derivativesAdenosineSynthesis methods
The invention discloses a preparation method of 5'-chlorine nucleoside belonging to medicinal chemistry technical field, which uses adenosine, guanosine, cytidine or uridine as raw material, adds triphenyl phosphine and hexachloroethane while the mol ratio of nucleoside / triphenyl phosphine / exachloroethane is 1:3-3.5:3-3.5, in one reactor to synthesize 5'-chlorine nucleoside. The preparation method has high yield, selective synthesis, simple operation, mild conditions, non pollution and support for industrial production. The 5'-chlorine nucleoside derivative is important intermediate of organic synthesis or drug synthesis, and the invention provides a synthesis method for 5'-chlorine nucleoside with high industrial value, which can accelerate the development and application of 5'-chlorine nucleoside.
Owner:ZHENGZHOU UNIV
Method for synthesizing cyclic adenosine monophosphate
ActiveCN109206465AReduce usageNovel design routeSugar derivativesSugar derivatives preparationAcetic anhydrideAcetylation
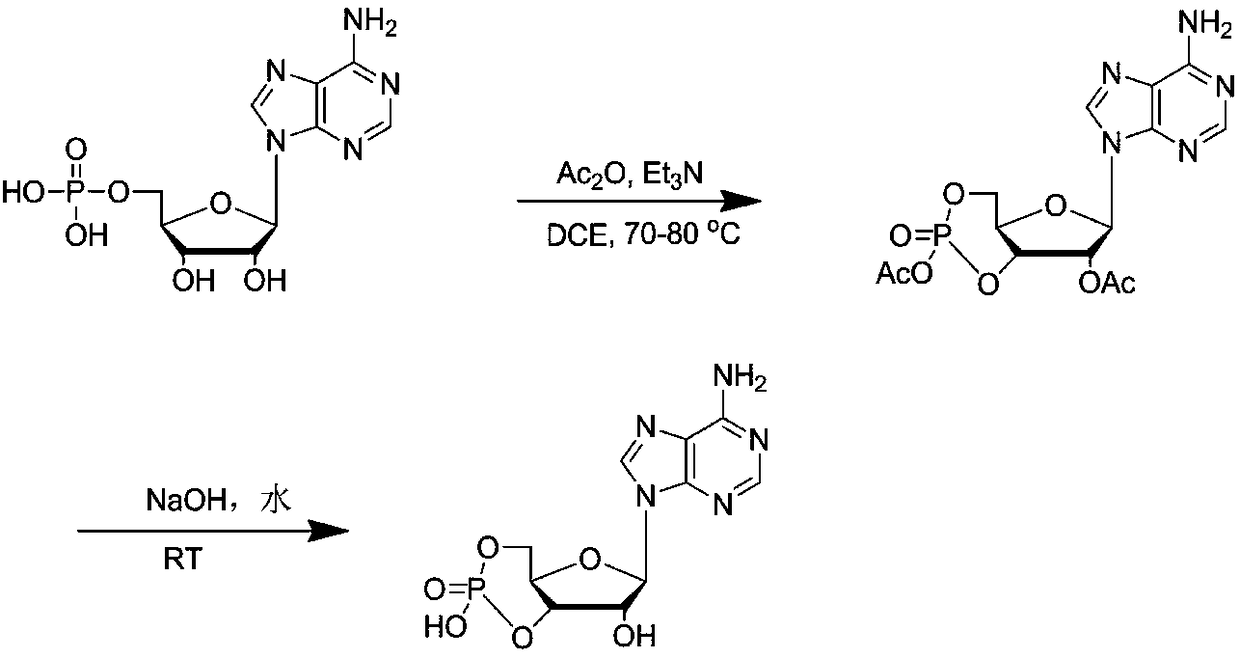
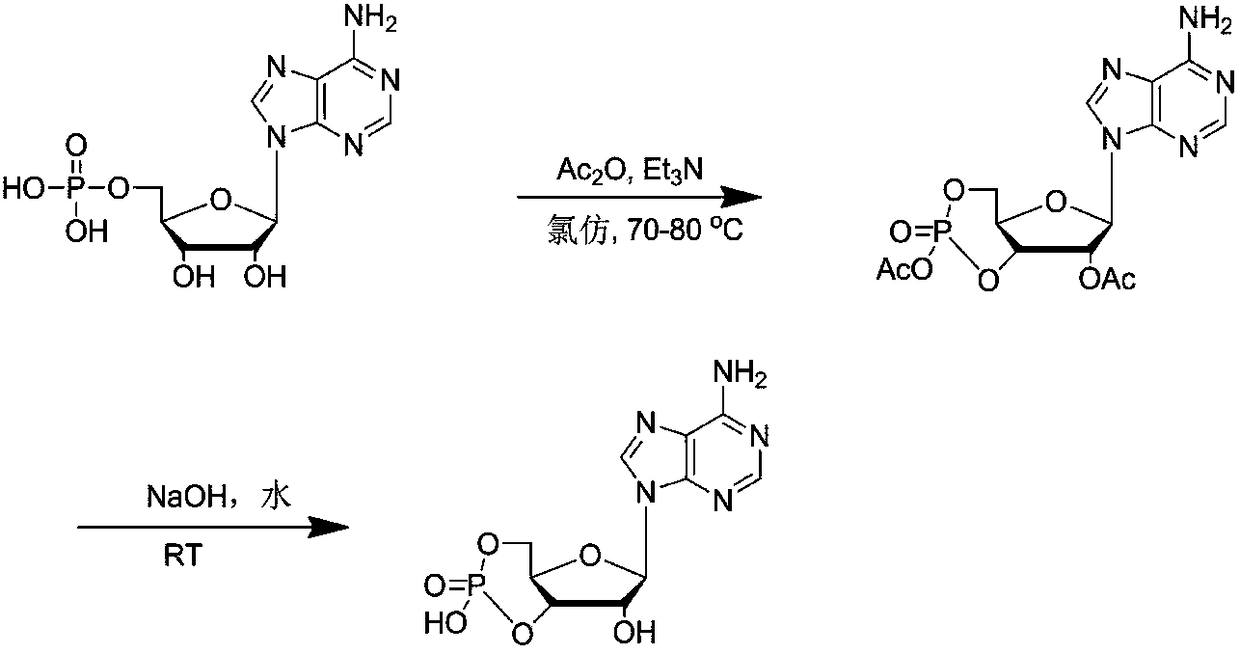
The invention discloses a method for synthesizing cyclic adenosine monophosphate, belonging to the field of synthesis of nucleosides in organic chemistry. The method comprises the following reaction steps: with adenylic acid as a raw material, performing one-step cyclization under the action of acetic anhydride so as to obtain acetylated cyclic adenosine monophosphate; and then performing alkalinehydrolysis so as to obtain cyclic adenosine monophosphate. The method only needs two steps of reaction in the whole process, is simple to operate, avoids usage of considerable solvents that are usedin traditional synthesis processes, has been verified in a kg-grade scale and has industrial application prospects.
Owner:TUOXIN GROUP +1
Synthesis and use of 2′-substituted-N6 -modified nucleosides
An improved method of preparing a sugar modified nucleoside analog includes a protocol in which a hydroxy group of a sugar is selectively deprotected and oxidized prior to nucleophilic modification of the corresponding carbonyl group. The modified sugar is then coupled to a heterocyclic base that is modified with a dual nucleophilic reagent in a further step that provides N6-modified adenosine analogs with high stereoselectivity.
Owner:VALEANT PHARMACEUTICALS NORTH AMERICA LLC
Targeted control of pests and pathogens by plant delivery of 2'f-ana-oligonucleotides
Herein is disclosed synthetic oligonucleotides comprising 2′F-ANA nucleosides that can be utilized to control plant-chewing and phloem-feeding insects, bacteria present in such insects, and bacteria present in plants. The novel approaches and materials provided herein allow for reduction of pesticide and antibiotic use without the need to create genetically modified plants.
Owner:AUM LIFETECH INC +1
Methods for selective N-9 glycosylation of purines
A process for providing regiospecific and highly stereoselective synthesis of 9-β anomeric purine nucleoside analogs is described. The introduction of the sugar moiety on to 6-(azolyl)-substituted purine bases is performed so that highly stereoselective formation of the β anomers of only the 9 position regioisomers of the purine nucleoside analogs (either D or L enantiomers) is obtained. This regiospecific and stereoselective introduction of the sugar moiety allows the synthesis of nucleoside analogs, and in particular 2′-deoxy, 3′-deoxy, 2′-deoxy-2′-halo-arabino and 2′,3′-dideoxy-2′-halo-threo purine nucleoside analogs, in high yields without formation of the 7-positional regioisomers. Processes for providing novel 6-(azolyl)purines for the regiospecific and highly stereoselective synthesis of 9-β anomeric purine nucleoside analogs are described. The compounds are drugs or intermediates to drugs.
Owner:BRIGHAM YOUNG UNIV
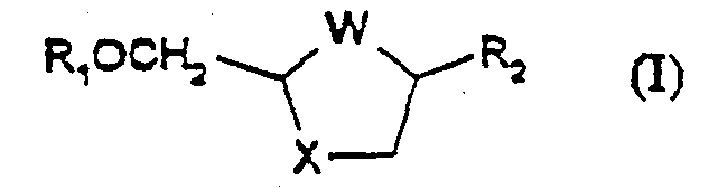
Process for the diastereoselective synthesis of nucleoside analogues
A diastereoselective process for the preparation of compounds of formula (I), wherein W is S, S=O, SO2, or O; X is S, S=O, SO2, or O; R1 is hydrogen or acyl, and R2 is a purine or pyrimidine base or an analogue or derivative thereof, is described.
Owner:GLAXO GRP LTD
Process for the diastereoselective synthesis of nucleoside analogues
A diastereoselective process for the preparation of compounds of formula (I), wherein W is S, S=O, SO2, or O; X is S, S=O, SO2, or O; R1 is hydrogen or acyl, and R2 is a purine or pyrimidine base or an analogue or derivative thereof, is described.
Owner:GLAXO GROUP LTD
Synthesis process of fluoronucleoside
InactiveCN106831915AGuaranteed to choose correctlyImprove securitySugar derivativesSugar derivatives preparationCytosineFiltration
The invention relates to a synthesis process of fluorinated nucleosides, comprising the following steps: step 1, refluxing cytosine and hexamethyldisilazane under the catalysis of ammonium sulfate, then adding isopropanol, and raising the temperature, Obtain a cytosine silyl ether protecting group solution; step 2, dissolve the glycosyl compound in isoamyl alcohol and stir, then add a catalyst and heat to obtain a glycosyl compound solution; step 3, drop the glycosyl compound into the cytosine silyl ether protecting group solution Add the glycosyl compound solution, after the dropwise addition, continue to keep warm for an hour, then filter with suction, add hydrochloric acid dropwise to the filtrate to precipitate a solid, and obtain an intermediate after drying; step 4, the intermediate Carry out the deprotection of the hydroxyl group to obtain the fluorinated nucleoside. The invention not only ensures the formation of the target intermediate configuration, but also greatly reduces the temperature, which not only reduces the requirements for equipment, improves the safety of operation, but also reduces energy consumption, which is beneficial to reduce costs, and is suitable for large-scale production applications .
Owner:上海泰坦科技股份有限公司
Method for compounding 2,6-dichloropurine nucleoside by using inosine as raw material
ActiveCN105418710AReduce usageRaw materials are easy to getSugar derivativesSugar derivatives preparationTrifluoromethanesulfonic anhydridePurine
The invention discloses a method for compounding 2,6-dichloropurine nucleoside by using inosine as a raw material. The method is characterized by using cheap inosine as the raw material; obtaining 6-chlorine triacetyl purine nucleoside through acetylation and chlorination reactions; then enabling the 6-chlorine triacetyl purine nucleoside to react with trifluoromethanesulfonic acid; introducing nitro in at a second site; finally completing two reactions of acetyl removing and nitro chlorination in an ethanol solution which is saturated by a hydrogen chloride gas, obtaining the 2,6-dichloropurine nucleoside, wherein the total yield is 63 percent. According to the method for compounding the 2,6-dichloropurine nucleoside by using the inosine as the raw material, disclosed by the invention, the raw materials are cheap and are easily obtained, a reagent of which the price is expensive and poisonous and harmful heavy metal catalysts are prevented from being used, and the yield is not obviously reduced when the reaction scale is enlarged to 200 g; a new compounding way is provided for compounding of the 2,6-dichloropurine nucleoside, and a potential application prospect is obtained.
Owner:XINXIANG UNIV
Synthesis of 2'-deoxy-l-nucleosides
This invention provides processes for the preparation of compounds having the structure: wherein X and Y are same or different, and H, OH, OR, SH, SR, NH2, NHR', or NR'R'' Z is H, F, Cl, Br, I, CN, or NH2. R is hydrogen, halogen, lower alkyl of C1-C6 or aralkyl, NO2, NH2, NHR', NR'R'', OH, OR, SH, SR, CN, CONH2, CSNH2, CO2H, CO2R', CH2CO2H, CH2CO2R', CH-CHR, CH2CH-CHR, or C-CR. R' and R'' are same or different, and lower alkyl of C1-C6. R13 is hydrogen, alkyl, acyl, phosphate (monophosphate, diphosphate, triphosphate, or stabilized phosphate) or silyl; and
Owner:法玛赛特有限公司
Synthesis of nucleosides
ActiveUS9109000B2Inhibition formationEasy to removeTin organic compoundsSugar derivativesMizoribinePurine
A process for the preparation of nucleosides, derivatives and analogues thereof by coupling reaction of a protected suitable nitrogeneous purine or pyrimidine base, a derivative or analogue thereof and a protected suitable sugar in the presence of SnCl4 comprising the removal of SnCl4 by adding DMSO directly into the reaction mixture is described. Preferably said process is used for the preparation of antiviral and antitumor agents having a nucleoside or nucleoside-like structure, still more preferably for the preparation of azacytidine, decitabine, chlorfarabine, cladribine, mizoribine. A residual tin content lower than 300 ppm is obtained with said process.
Owner:FARMABIOS
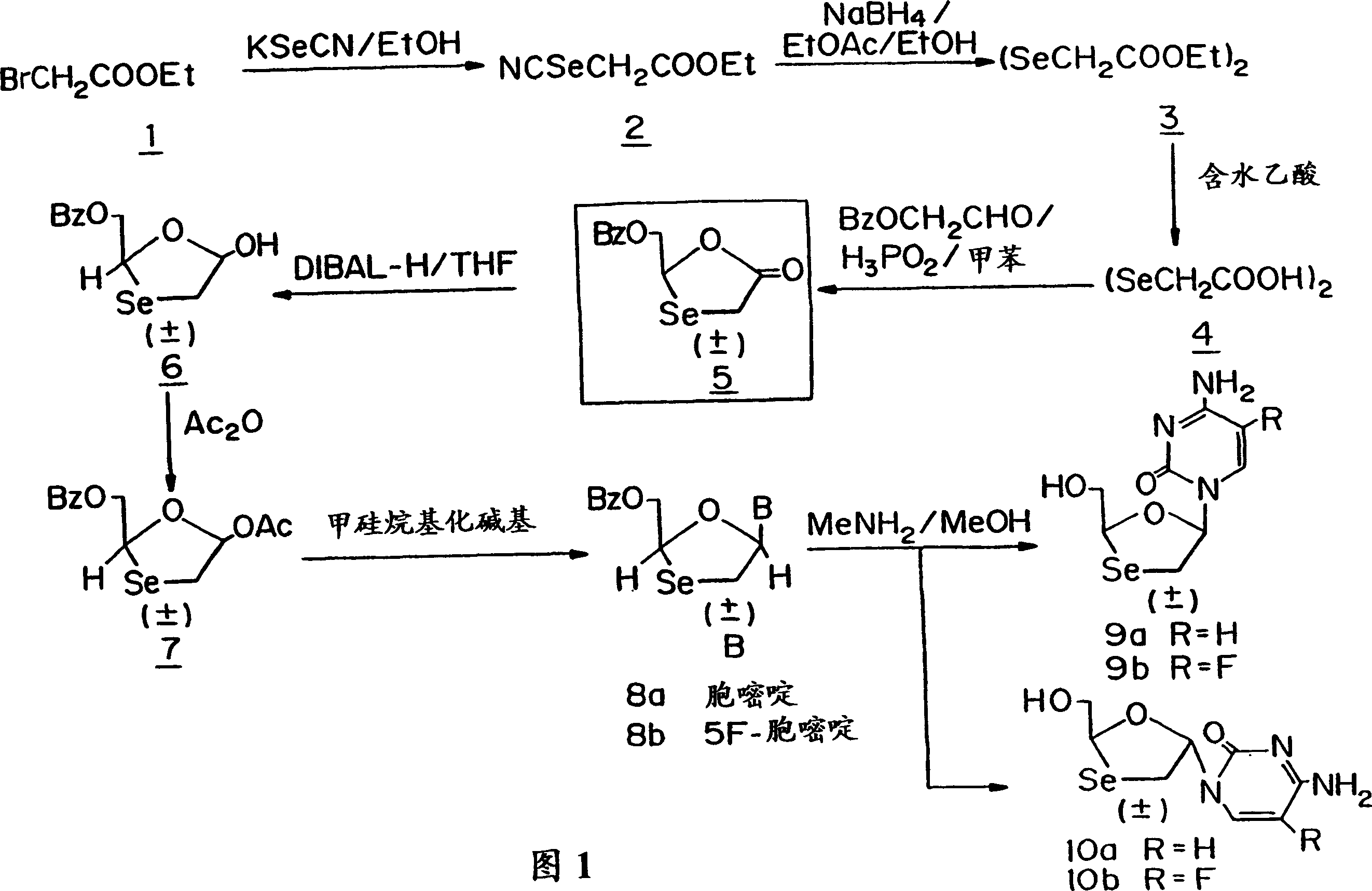
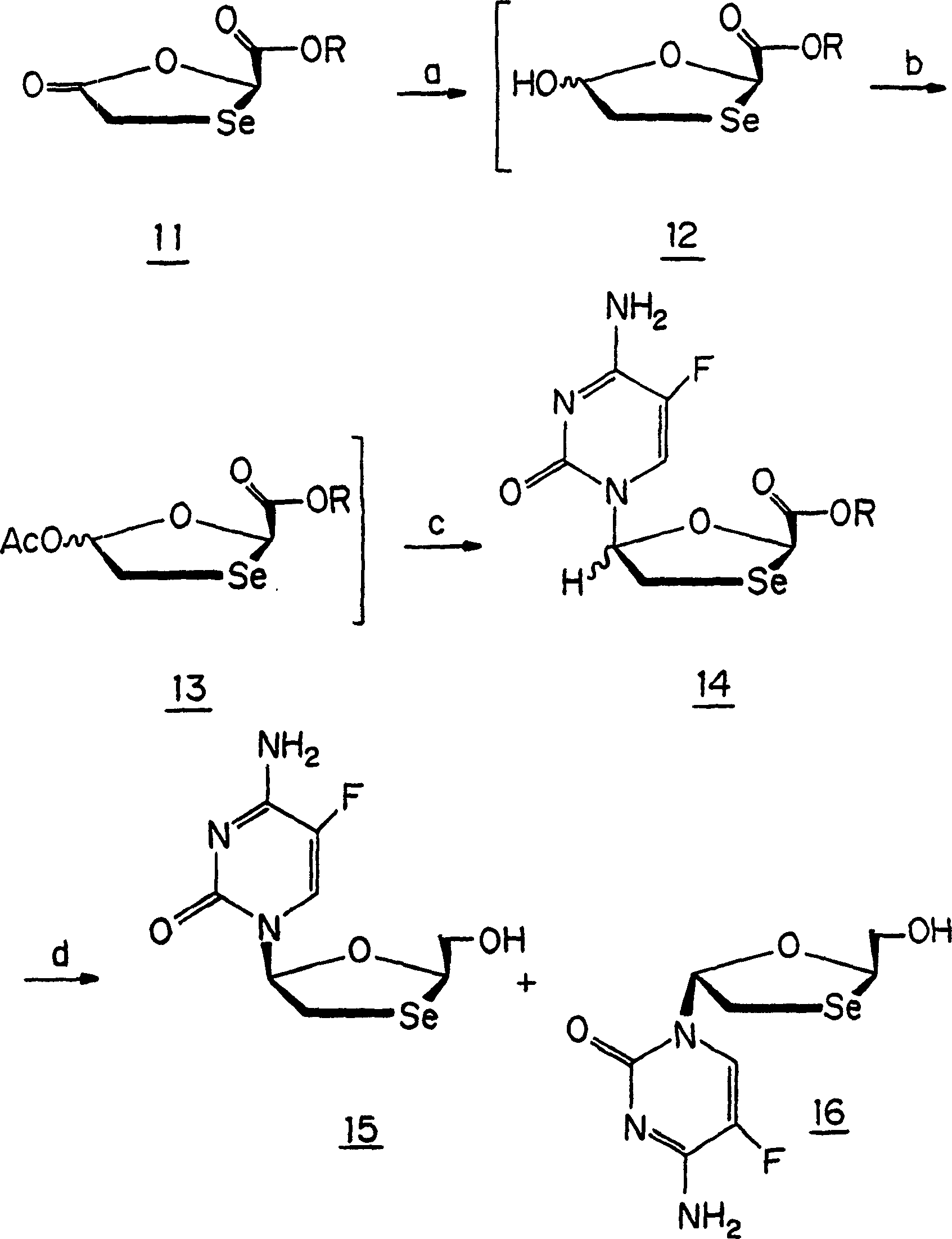
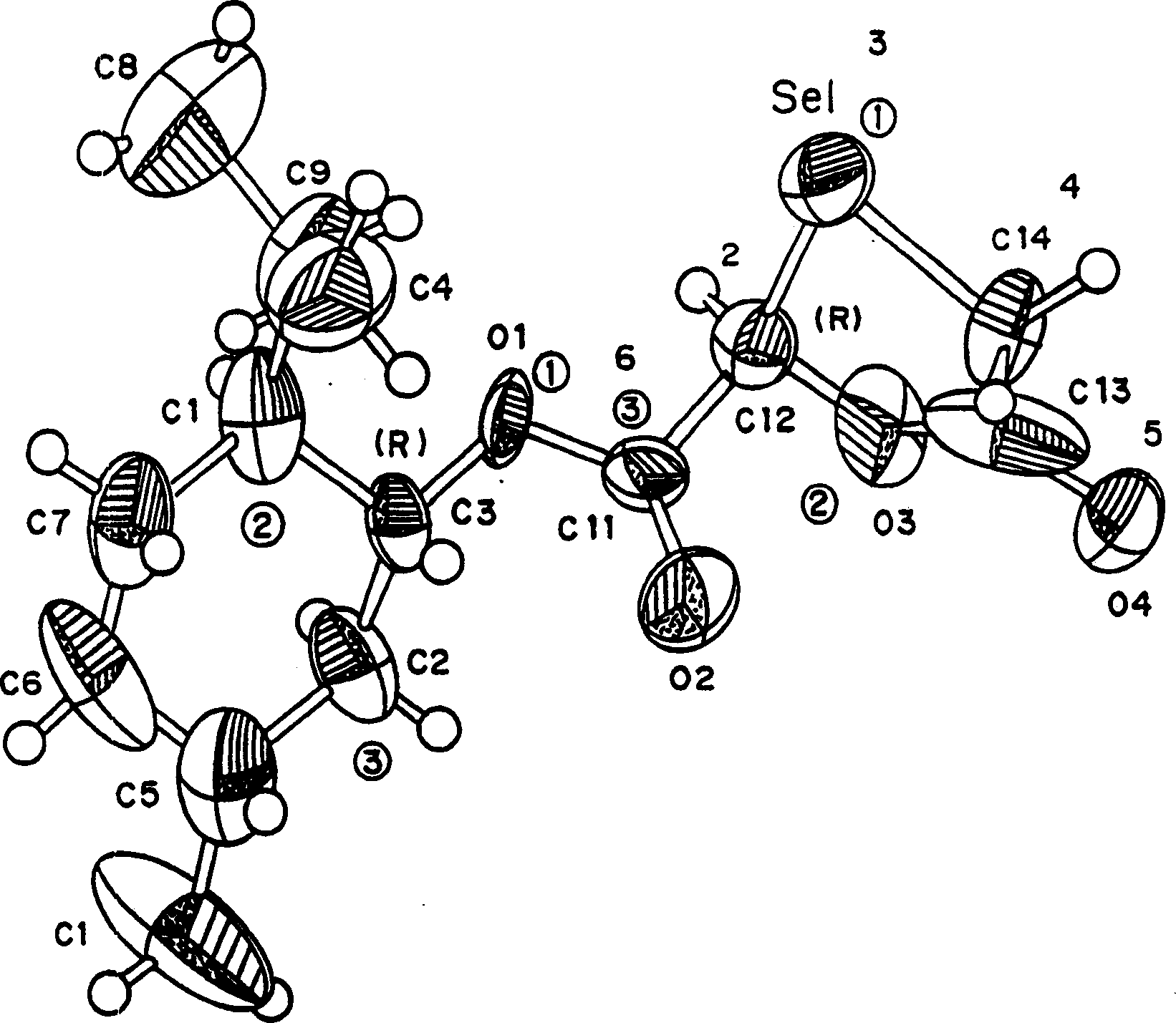
Synthesis, anti-human immunodeficiency virus and anti-hepatitis B virus activities of 1-3-oxaselenolane nucleosides
InactiveCN1196701CHalogenated hydrocarbon active ingredientsGroup 5/15 element organic compoundsSynthesis of nucleosidesHepatitis b viral
A method and composition for the treatment of HIV infection, HBV infection, or abnormal cellular proliferation in humans and other host animals is disclosed that includes the administration of an effective amount of a 1,3-oxaselenolane nucleoside or a pharmaceutically acceptable salt thereof, optionally in a pharmaceutically acceptable carrier.
Owner:EMORY UNIVERSITY
Compound and preparation method thereof, and preparation method of nucleoside oligophosphate
PendingCN113621012AStable generationQuick buildSugar derivativesSugar derivatives preparationPhosphoric acidCombinatorial chemistry
The invention relates to the technical field of nucleotide synthesis, in particular to a compound and a preparation method thereof, and a preparation method of nucleoside oligophosphate. The preparation method of the compound comprises the following steps: putting a nucleoside phosphate raw material, imidazole and an activating agent into a first solvent for a first reaction. According to the invention, the water-soluble raw material and the activating agent are subjected to a reaction to prepare the compound, and the compound can be used as a raw material for synthesizing nucleoside oligophosphate, so that rapid and stable synthesis of nucleoside oligophosphate is facilitated. The phosphate raw material and the compound prepared in the invention are subjected to a second reaction in a second solvent, and then desalination is performed to prepare nucleoside oligophosphate; compared with the prior art, triethylamine conversion does not need to be carried out on the nucleoside phosphate raw material, the utilization rate of the nucleoside phosphate raw material is increased, and the reaction period is shortened.
Owner:SHANGHAI ZHAOWEI TECH DEV +1
Targeted control of pests and pathogens by plant delivery of 2'F-ANA-oligonucleotides
Owner:AUM LIFETECH INC +1
Preparing method of 1,2,3-tris-O-acetyl-5-deoxy-beta-D-ribose
PendingCN108558960AConvenient sourceEase of industrial productionEsterified saccharide compoundsSugar derivativesAcetic anhydrideSynthesis methods
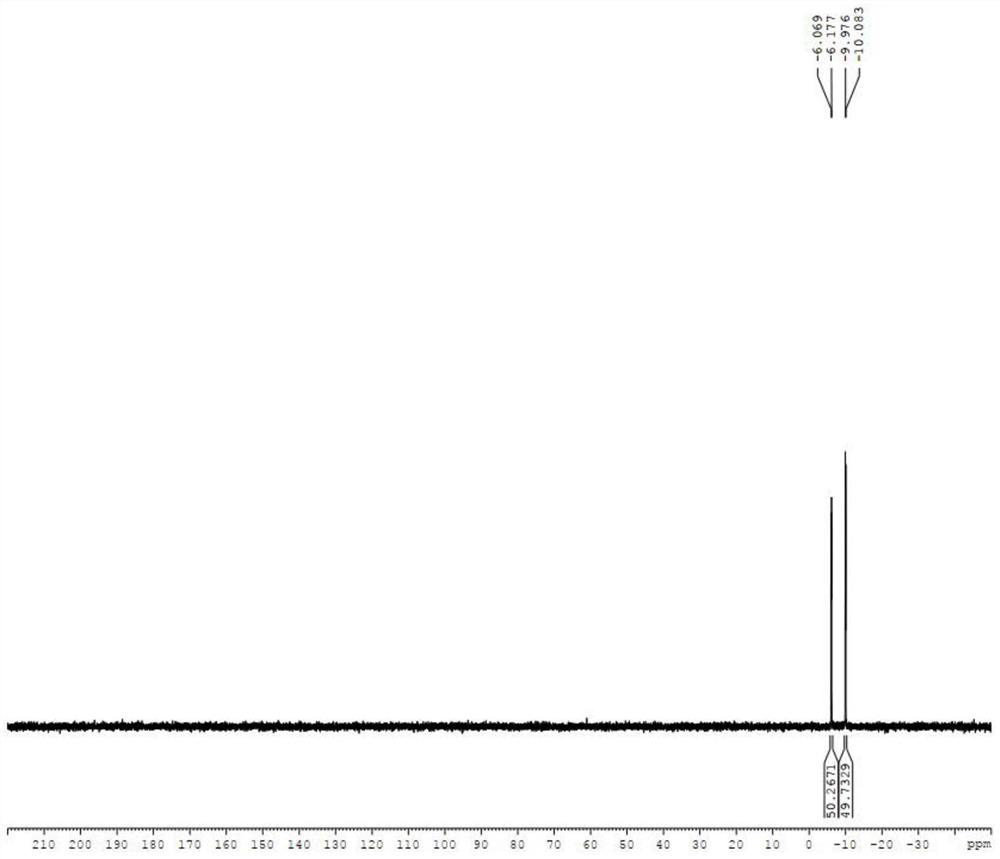
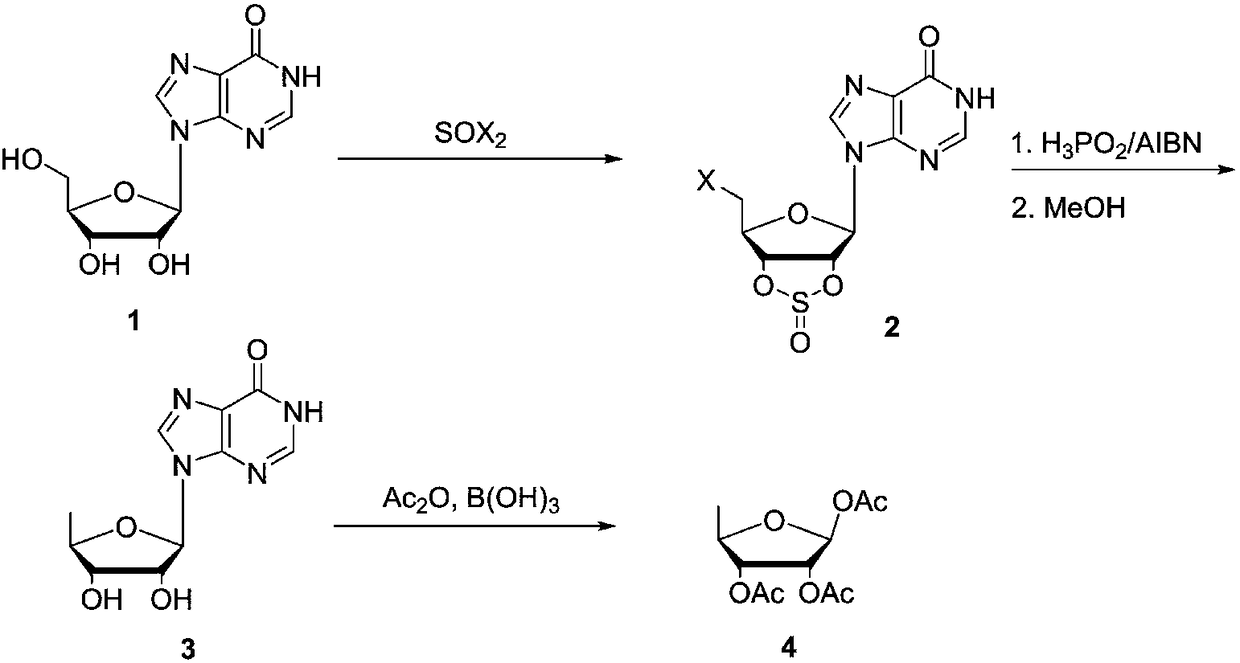
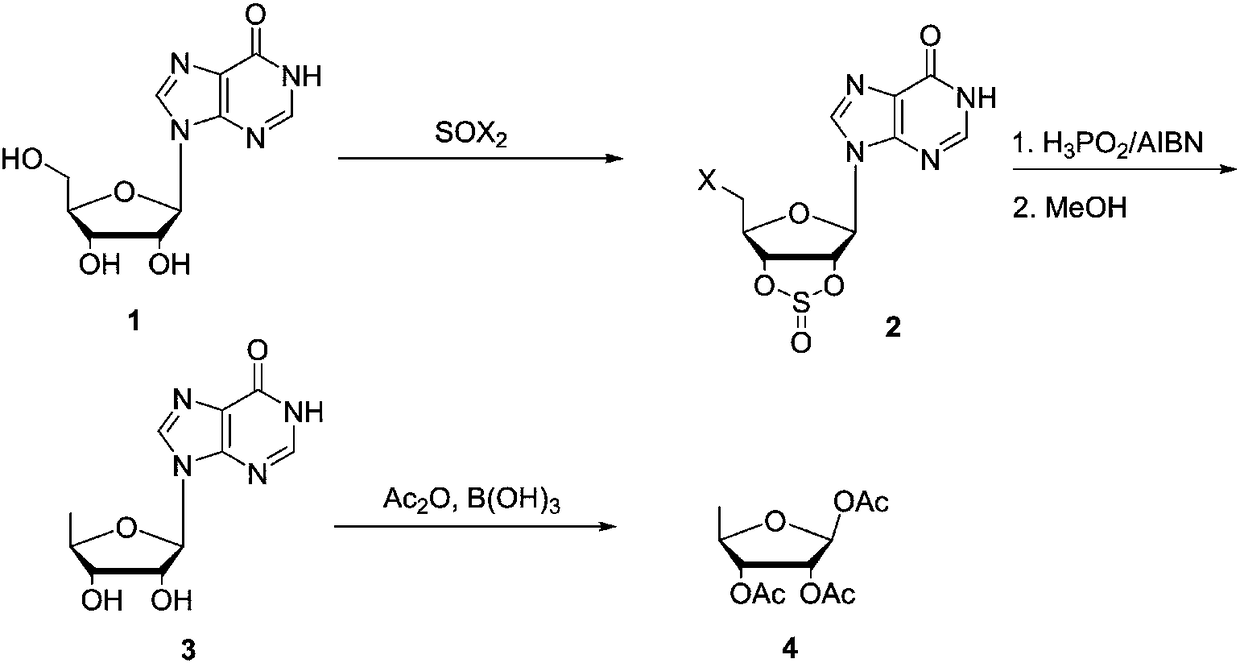
The invention discloses a preparing method of 1,2,3-tris-O-acetyl-5-deoxy-beta-D-ribose, and belongs to the field of synthesis of nucleosides in organic chemistry. The preparing method includes the steps of making inosine 1 react with halogenated sulfoxide to obtain a compound 2, reducing the compound 2 under the conditions of hypophosphorous acid and AIBN, adding methyl alcohol for deprotection to obtain a compound 3, and making the compound 3 react in acetic anhydride under the catalysis of inorganic boric acid to obtain 1,2,3-tris-O-acetyl-5-deoxy-beta-D-ribose. The synthesis method is lowin raw material price, short in step and easy to industrially produce and has industrial application prospects.
Owner:TUOXIN GROUP +1
Synthesis of 2'-deoxy-l-nucleosides
This invention provides processes for the preparation of compounds having the structure: wherein X and Y are same or different, and H, OH, OR, SH, SR, NH2, NHR', or NR'R'' Z is H, F, Cl, Br, I, CN, or NH2. R is hydrogen, halogen, lower alkyl of C1-C6 or aralkyl, NO2, NH2, NHR', NR'R'', OH, OR, SH, SR, CN, CONH2, CSNH2, CO2H, CO2R', CH2CO2H, CH2CO2R', CH-CHR, CH2CH-CHR, or C-CR. R' and R'' are same or different, and lower alkyl of C1-C6. R13 is hydrogen, alkyl, acyl, phosphate (monophosphate, diphosphate, triphosphate, or stabilized phosphate) or silyl; and
Owner:法玛赛特有限公司
Popular searches
Features
- R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
Why Patsnap Eureka
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Social media
Patsnap Eureka Blog
Learn More Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com