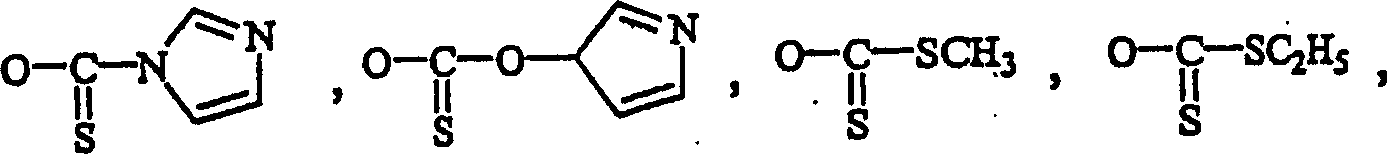Synthesis of 2'-deoxy-l-nucleosides
A technology of nucleosides and compounds, applied in the field of ,-H., can solve the problems of cell replication or cell metabolism interruption, inability to treat disseminated tumor diseases, and inability to treat tumors, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
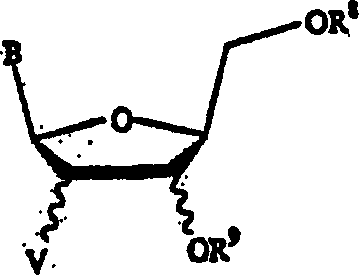
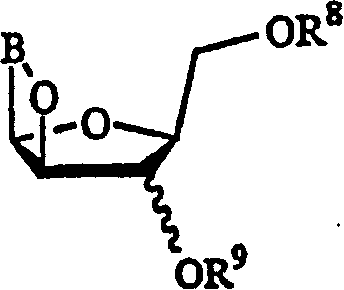
[0065] The present invention discloses herein a process for the preparation of the compound of formula (A).
[0066]
[0067] in
[0068] X and Y are independently hydrogen, OH, OR 1 , SH, SR 1 , NH 2 , NHR 1 or NR 1 R 2 ;
[0069] Z is hydrogen, halogen, CN or NH 2 ;
[0070] R is hydrogen, lower alkyl, aralkyl, halogen, NO 2 , NH 2 , NHR 3 、NR 3 R 4 , OH, OR 3 , SH, SR 3 , CN, CONH 2 、CSNH 2 , CO 2 H, CO 2 R 3 、CH 2 CO 2 H, CH 2 CO 2 R 3 , CH=CHR 3 、CH 2 CH=CHR 3 or C=CR 3 ;
[0071] R 1 , R 2 , R 3 and R 4 independently lower alkyl such as methyl, ethyl, propyl, butyl, and alkyl having 6 or less carbons, including cyclic, branched or linear, unsubstituted or substituted alkyl, wherein Alkyl groups may be substituted by one, two or more groups including, but not limited to, amino, carboxyl, hydroxyl and phenyl;
[0072] R 13 is hydrogen, alkyl, acyl, phosphate (monophosphate, diphosphate, triphosphate or stabilized phosphate) or silyl. ...
Embodiment 1
[0630] 1-O-acetyl-2,3,5-tri-O-benzoyl-β-L-ribofuranose (1,R 1 =Ac, R 2 =Bz)
[0631] 1-O-acetyl-2,3,5-tri-O-benzoyl-β-D-ribofuranose (1,R 1 =Ac, R 2 =Bz), the compound is prepared from L-ribose. A mixture of L-ribose (150 g, 1.0 mol) in methanol (2.5 L) containing 1% hydrochloric acid was stirred for 2 hours, then neutralized with pyridine (250 mL). The mixture was concentrated in vacuo and the residue was dissolved in pyridine (1 L). Benzoyl chloride (385 mL, 3.3 mol) was added dropwise to the solution during cooling to 0°C. After overnight at room temperature, the mixture was concentrated in vacuo at 35-40 °C, and the residue was dissolved in ethyl acetate (1.5 L). With cold water (2×0.5L), 1N H 2 SO 4 (3×0.5mL), water (0.5L) and saturated sodium bicarbonate (2×0.5mL) washed the organic solution successively, dried over magnesium sulfate, concentrated in vacuo into a slurry, then dissolved in glacial acetic acid (200mL) and acetic anhydride ( 0.5L) in the mixture. ...
Embodiment 2
[0633] 2,3,5-tri-O-benzoyl-D-ribofuranosyl bromide (2, X'=Br, R 2 =Bz)
[0634] Hydrogen bromide was bubbled into a solution of compound 1 (25.2 g, 0.05 mol) in ice-cold dichloromethane (150 mL) over 15 minutes. After standing at 0°C for 1 hour and at room temperature for 15 minutes, the solution was concentrated in vacuo. Continue azeotropic distillation with toluene (25mL×5) to remove traces of hydrogen bromide. The syrupy residue (2) is immediately condensed with the appropriate purine or pyrimidine. of the slurry 1 The H-NMR spectrum includes a singlet δ 6.5 (H-1, β-anomer) and a doublet 6.9 (H-1α-anomer, J 1,2 = 4.4Hz). α / β is about 3:2.
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com