Method for synthesizing positive electron radioactive imaging agent labeled precursor thymidine derivative
A technology of thymidine derivatives and synthetic methods, which is applied in the field of chemical synthesis, can solve the problems of complex operation, high cost, and long cyclization reaction time, and achieve the effect of simple operation, low cost, and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
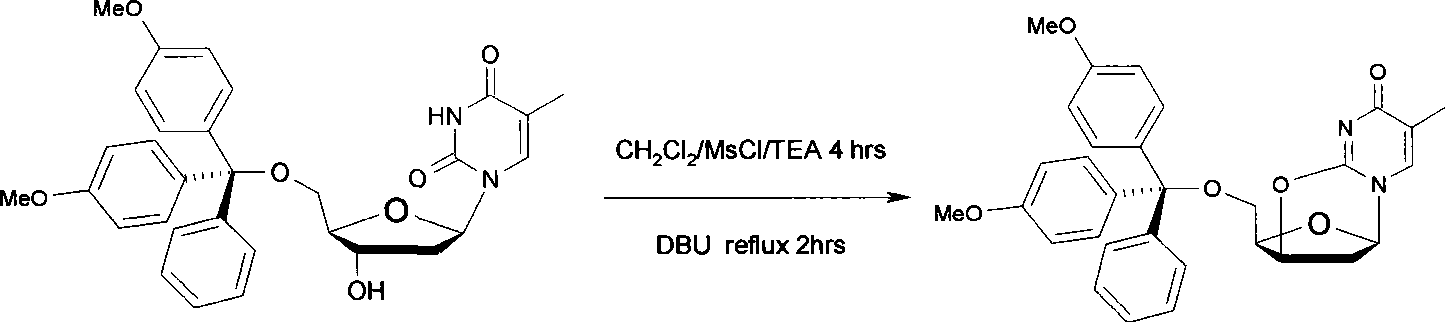
[0027] (1) Dissolve 1.5 g (6 mmol) of thymidine in 25 mL of anhydrous pyridine, stir magnetically, add 2.54 g (7.5 mmol) of 4,4'-dimethoxytrityl chloride under nitrogen protection, and (20-25°C) to react for 2 hours.
[0028] (2) The mixture was cooled to 0°C, and 1.16ml (15mmol) of methanesulfonyl chloride was added, then the temperature of the mixture was raised to room temperature (20-25°C), and magnetically stirred for 2 hours.
[0029] (3) The mixture was concentrated to dryness by rotary evaporation, the residue was dissolved in 60 mL of dichloromethane, 20 mL of 1% aqueous sodium hydroxide solution (w / v) was added, stirred well, and reacted for 30 minutes.
[0030] (4) Take the dichloromethane layer, wash with water 3 times and collect the organic phase. The organic phase was dried by adding anhydrous sodium sulfate, concentrated, and separated by silica gel column chromatography (eluent: ethyl acetate: n-hexane = 3: 1) to obtain 2.36 g of a white solid with a yield of...
Embodiment 2
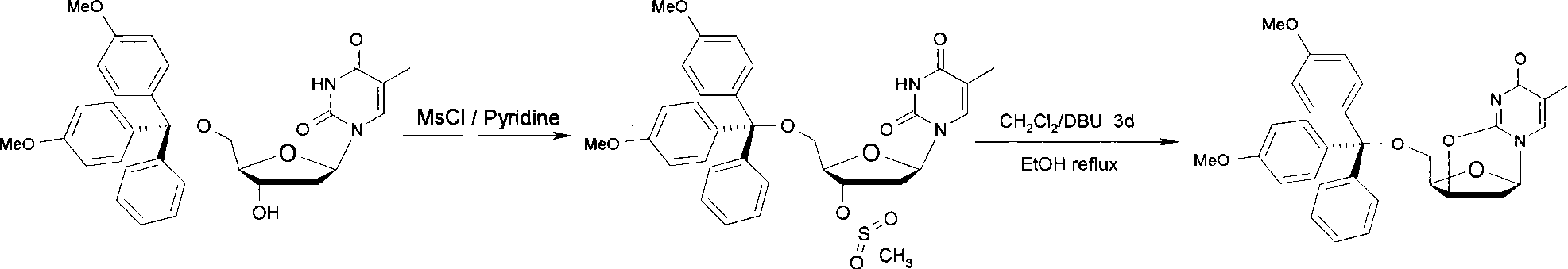
[0032] (1) Dissolve 1.5 g (6 mmol) of thymidine in 30 mL of anhydrous pyridine, stir magnetically, and add 2.54 g (7.5 mmol) of 4,4'-dimethoxytrityl chloride under nitrogen protection, 30 °C for 3 hours.
[0033] (2) The mixture was cooled to 0° C., 1.16 ml (15 mmol) of methanesulfonyl chloride was added, and then the temperature of the mixture was raised to 30° C., and magnetically stirred for 3 hours.
[0034](3) Concentrate the mixture to dryness by rotary evaporation, dissolve the residue in 60 mL of dichloromethane, add 30 mL of 10% aqueous sodium bicarbonate solution, stir well, and react for 30 minutes.
[0035] (4) Take the dichloromethane layer, wash with water 3 times and collect the organic phase. The organic phase was dried by adding anhydrous sodium sulfate, concentrated, and separated by silica gel column chromatography (eluent: ethyl acetate: n-hexane = 3: 1) to obtain 2.81 g of a white solid with a yield of 89% and a purity of more than 98% ( high performance...
Embodiment 3
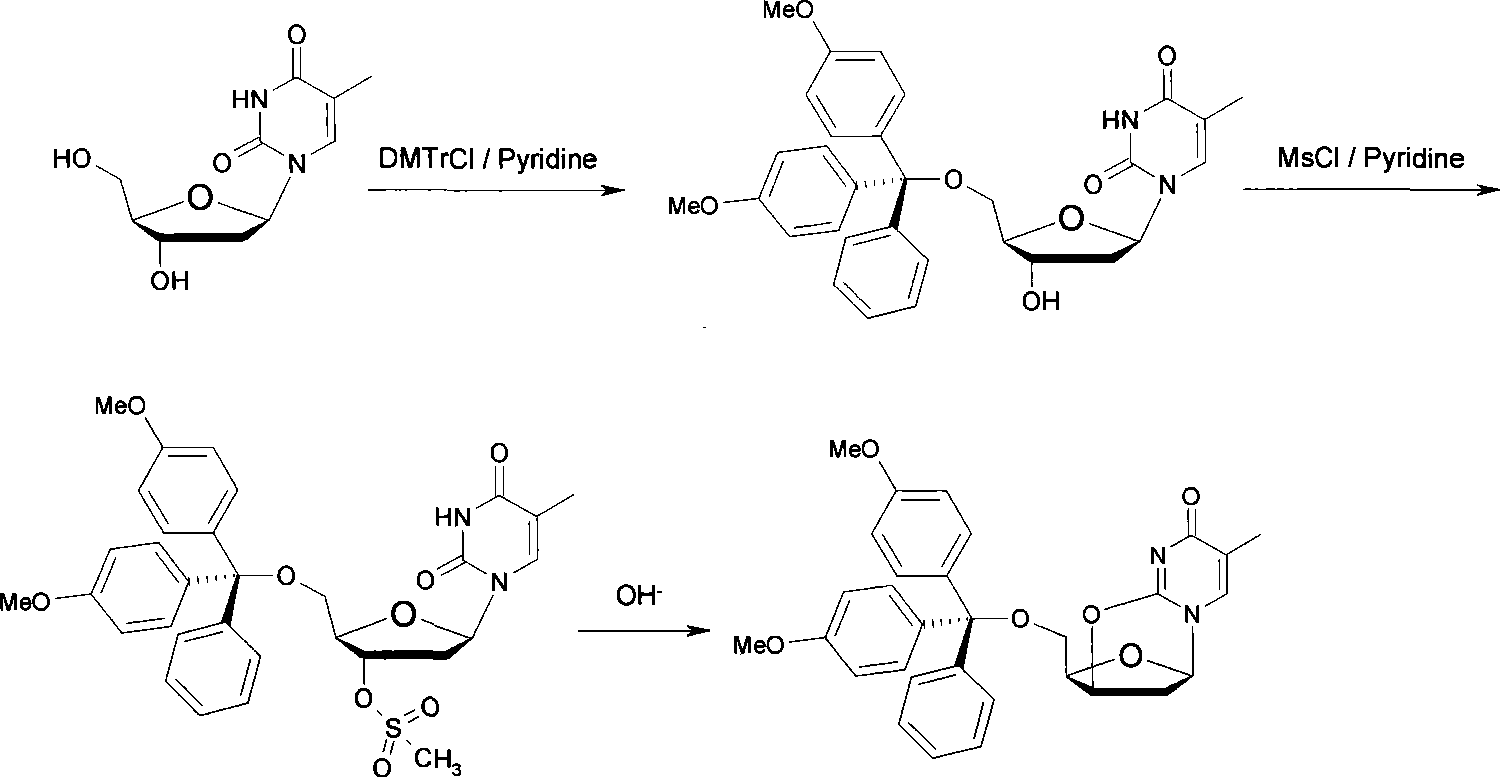
[0037] (1) Take thymidine 1.5g (6mmol) and dissolve it in 30mL anhydrous pyridine, stir it magnetically, add 4,4'-dimethoxytrityl chloride 3.05g (9mmol) under the protection of nitrogen, room temperature ( 20~25°C) for 3 hours.
[0038] (2) Cool the mixture to 0°C, add 1.55ml (20mmol) of methanesulfonyl chloride, then raise the temperature of the mixture to room temperature (20-25°C), and stir magnetically for 3 hours.
[0039] (3) The mixture was concentrated to dryness by rotary evaporation, the residue was dissolved in 50 mL of ethyl acetate, 25 mL of 20% sodium carbonate aqueous solution was added, fully stirred, and reacted for 45 minutes.
[0040] (4) Take the ethyl acetate layer, wash with water for 3 times and collect the organic phase. The organic phase was dried by adding anhydrous sodium sulfate, concentrated, and separated by silica gel column chromatography (eluent: ethyl acetate: n-hexane = 3: 1) to obtain 1.93 g of a white solid with a yield of 61% and a purity...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com