Stereoselective synthesis of beta-nucleosides
A technology of nucleoside compounds and compounds, applied in drug combination, organic chemistry, antineoplastic drugs, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
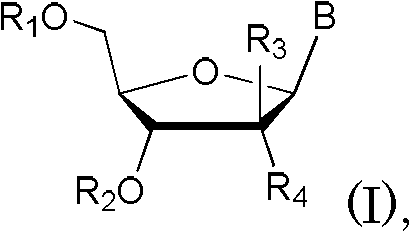
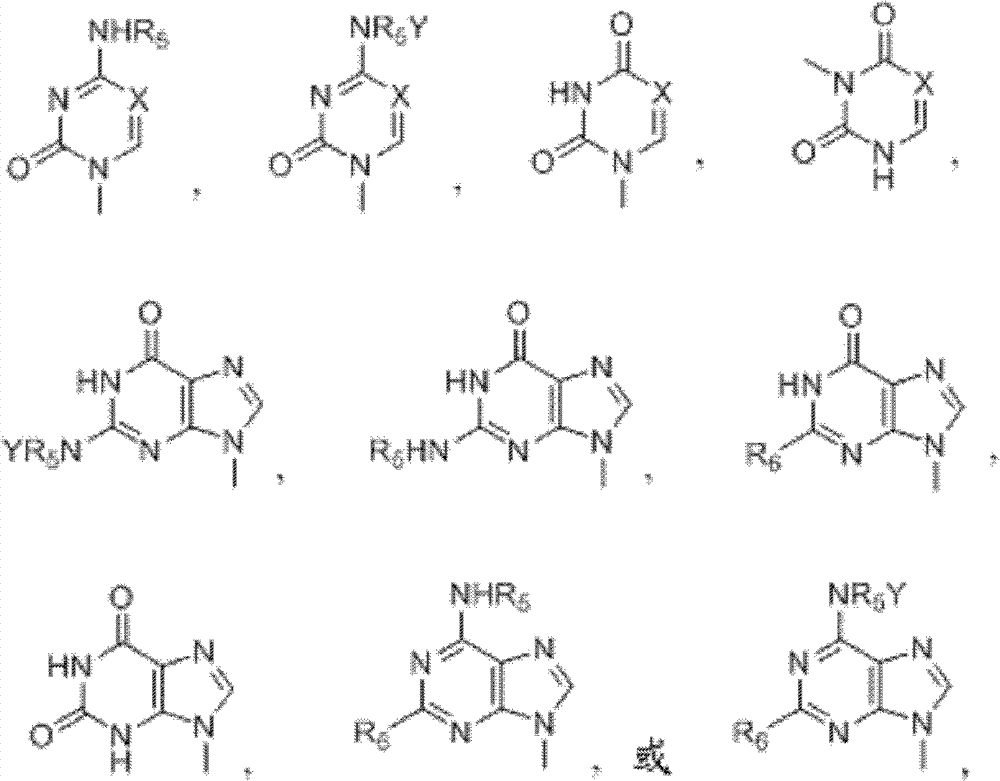
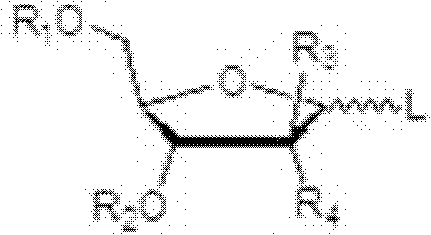
[0044] The present invention relates to an efficient method for the stereoselective synthesis of 2'-deoxynucleosides, more specifically gemcitabine, and novel intermediates produced in this method.
[0045] Conventional chemical transformations can be used to practice the invention. Those skilled in the art can determine the appropriate chemicals, solvents, protecting groups and reaction conditions for this transformation. Relevant information is described, for example, in R. Larock, Comprehensive Organic Transformations, VCH Publishers (1989); T.W. Greene and P.G.M. Wuts, Protective Groups in Organic Synthesis, 3rd edition, John Wiley and Sons (1999); L. Fieser and M. . Fieser, Fieser and Fieser's Reagents for Organic Synthesis, John Wiley and Sons (1994); with L. Paquette ed. Encyclopedia of Reagents for Organic Synthesis, John Wiley and Sons (1995) and other editions of these. Embodiments of the methods of the invention are described herein for purposes of illustration.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com