Compound and preparation method thereof, and preparation method of nucleoside oligophosphate
A compound and nucleoside base technology, applied in the field of nucleotide synthesis, can solve problems such as complex synthesis steps, long reaction cycle, and unstable synthesis process, and achieve the effects of shortening the reaction cycle, rapid production, and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0066] The present application provides a preparation method of a compound, comprising placing nucleoside phosphate raw materials, imidazole and an activator in a first solvent to perform a first reaction. Wherein, the structural formula of activator is as follows:
[0067]
[0068] In the structural formula of the activator: Y is selected from chlorine, bromine and PF 6 at least one of; R 5 selected from hydrogen and C 1 -C 4 At least one of the alkyl groups; R 6 selected from hydrogen, chlorine, bromine, C 1 -C 4 Alkyl and C 1 -C 4 At least one of the alkoxy groups; R 7 for C 1 -C 4 of alkyl.
[0069] As an example, in the structural formula of the activator, R 5 , R 6 and R 7 each independently selected from C 1 -C 4 In the case of an alkyl group, the number of carbon atoms in the alkyl group can be 1, 2, 3 or 4; in the structural formula of the activator, R 6 from C 1 -C 4 In the case of an alkoxy group, the number of carbon atoms in the alkoxy group ...
Embodiment 1
[0176] This embodiment provides a compound and its preparation method.
[0177]The preparation method of the compound: Dissolve nucleoside sodium monophosphate (5.0g) in 100mL of water and acetonitrile mixed solution (volume ratio of water and acetonitrile is 0.01), add activator 2-chloro-1,3-dimethyl Imidazole hexafluorophosphate (34.3g) and imidazole (1.7g) were reacted at 2°C for 0.5h. After the completion of the reaction was monitored by HPLC, the acetonitrile was removed by high vacuum concentration and freeze-dried at -80°C to obtain the compound product (3.8 g, 71%). Wherein, the structural formula of sodium nucleoside monophosphate is as follows:
[0178]
[0179] The structural formula of the obtained compound is as follows:
[0180]
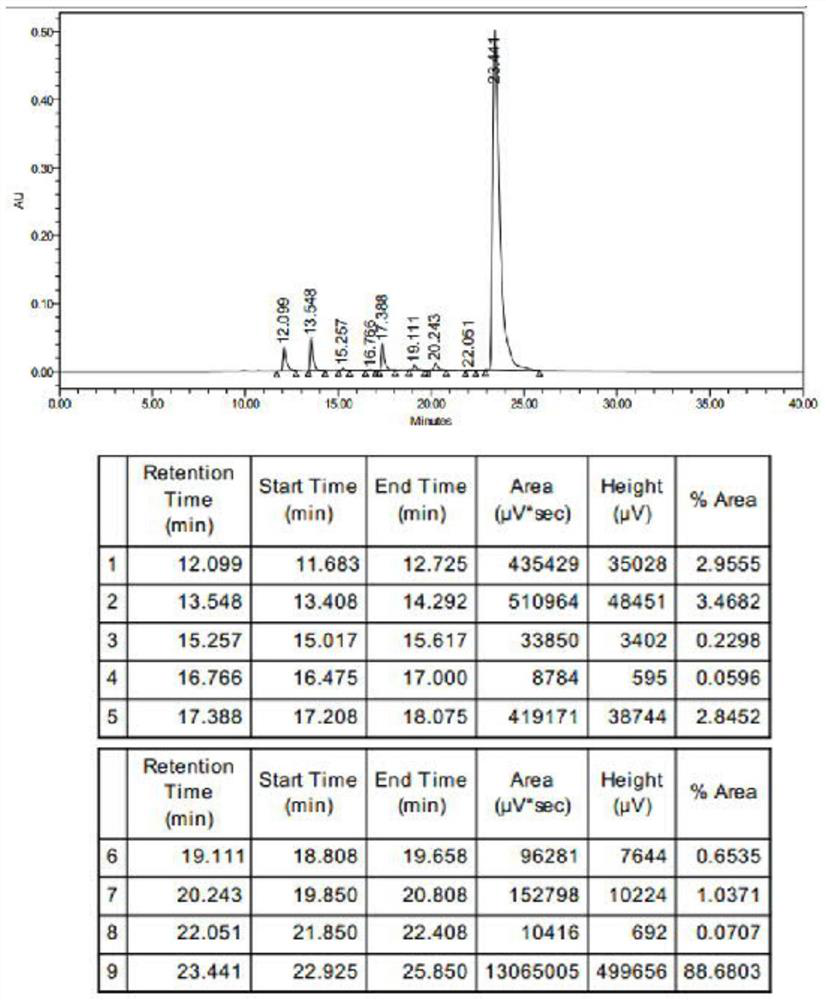
[0181] The compound product that above-mentioned reaction is made carries out HPLC (high performance liquid chromatography) characterization, and characterization result is as follows figure 1 shown. From figure 1 It can be s...
Embodiment 2
[0183] This example provides a preparation method of nucleoside triphosphates.
[0184] Compound (3.5g) was dissolved in 25mL of water, sodium pyrophosphate (Na 4 P 2 o 7 , 4.3g), reacted at 2°C for 4h. After HPLC monitors that the reaction is complete, add deionized water (3.0 L) to dilute, and purify with 80 mL of anion exchange resin, and use ammonium acetate / ammonium bicarbonate / triethylamine-carbonic acid buffer and deionized water as the mobile phase. The purified product was desalted with C18 and directly freeze-dried at -80°C to obtain the nucleoside triphosphate product (2 g, 47.6%). Wherein, the structural formula of compound is as follows:
[0185]
[0186] The structural formula of the nucleoside triphosphate product is as follows:
[0187]
PUM
| Property | Measurement | Unit |
|---|---|---|
| Boiling point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com