Synthesis, anti-human immunodeficiency virus and anti-hepatitis B virus activities of 1-3-oxaselenolane nucleosides
A technology of oxyselenosides and oxyselenosides, which is applied in the field of synthesizing nucleosides and can solve problems such as difficulties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0073] Preparation of II Active Compounds
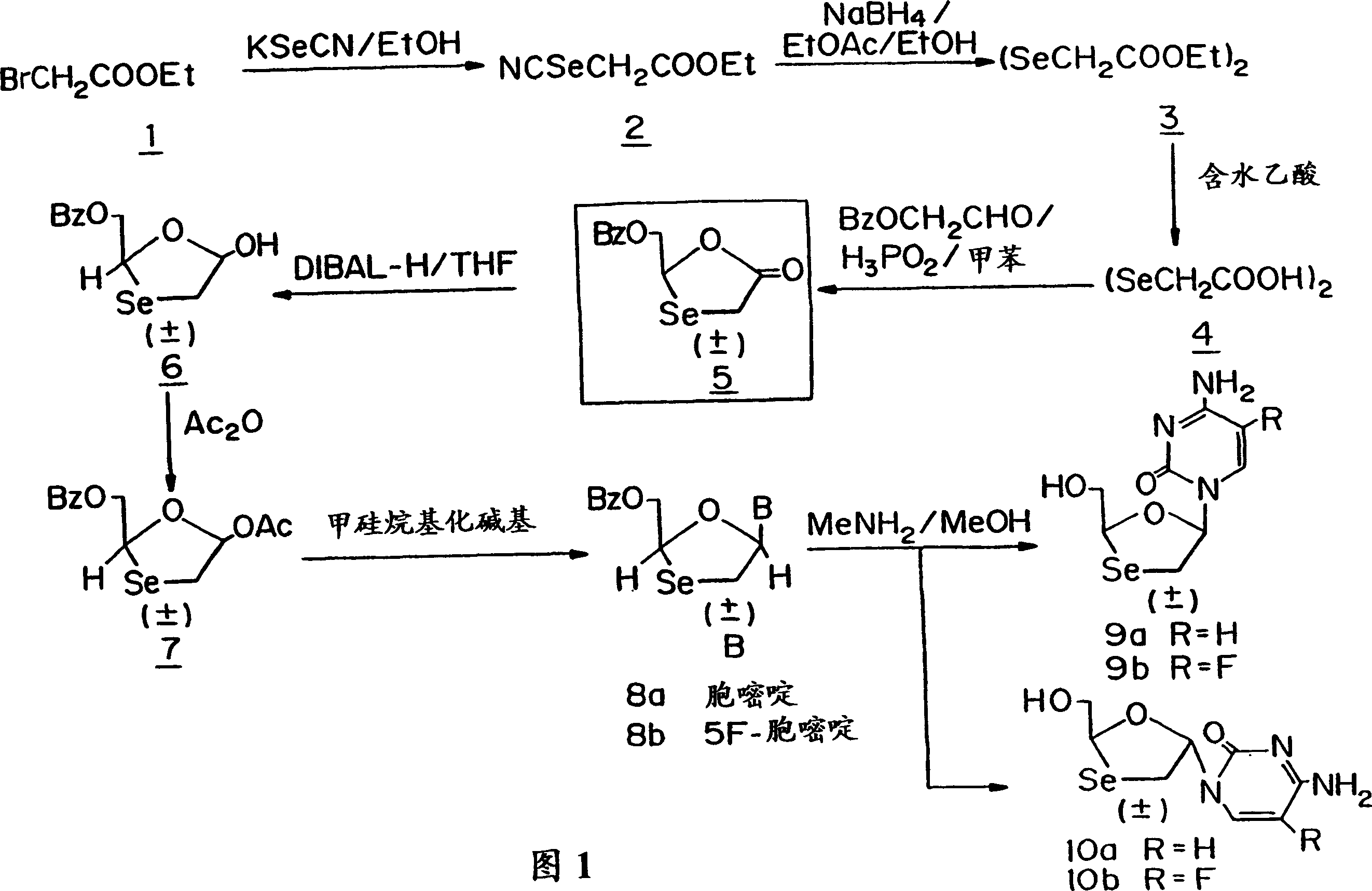
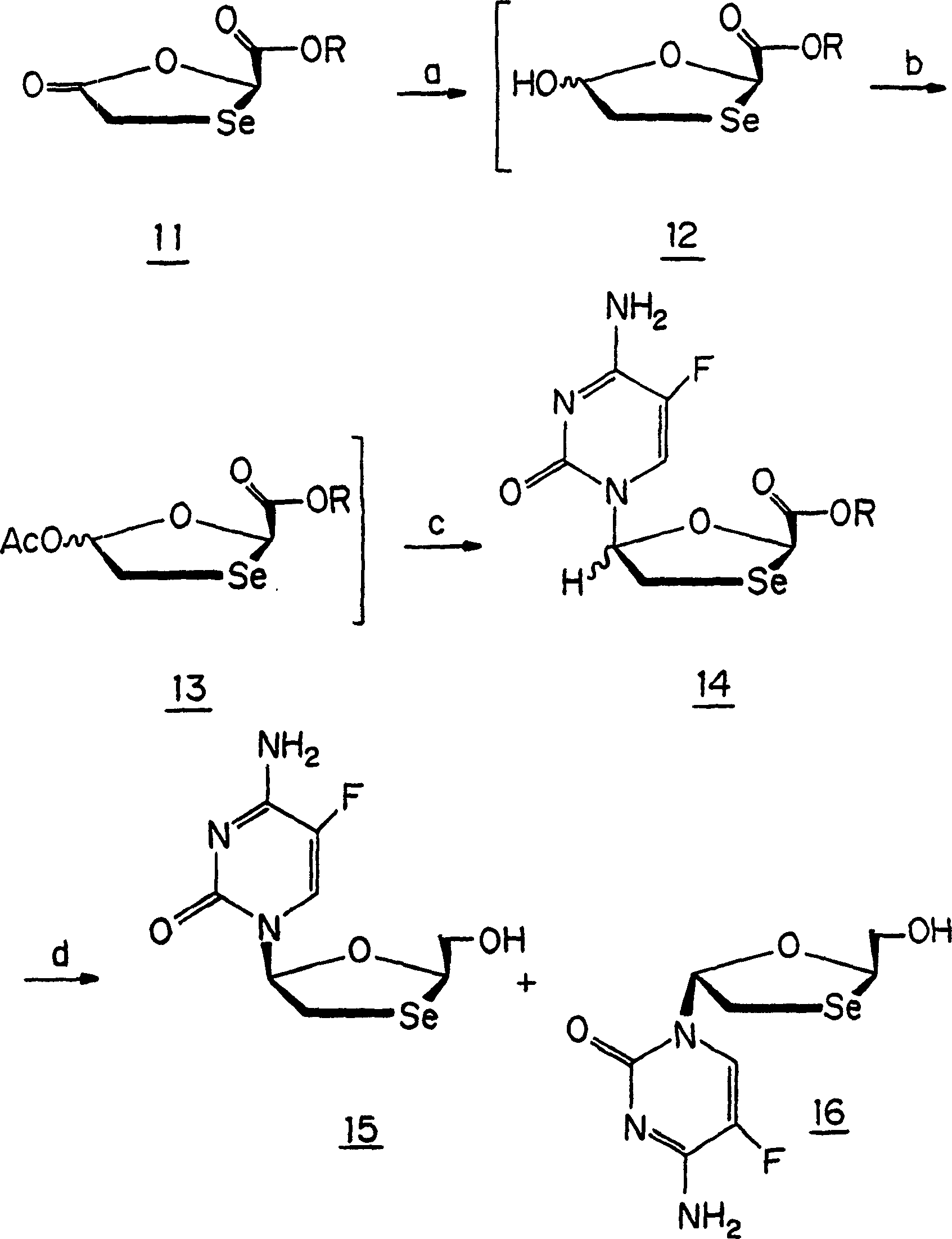
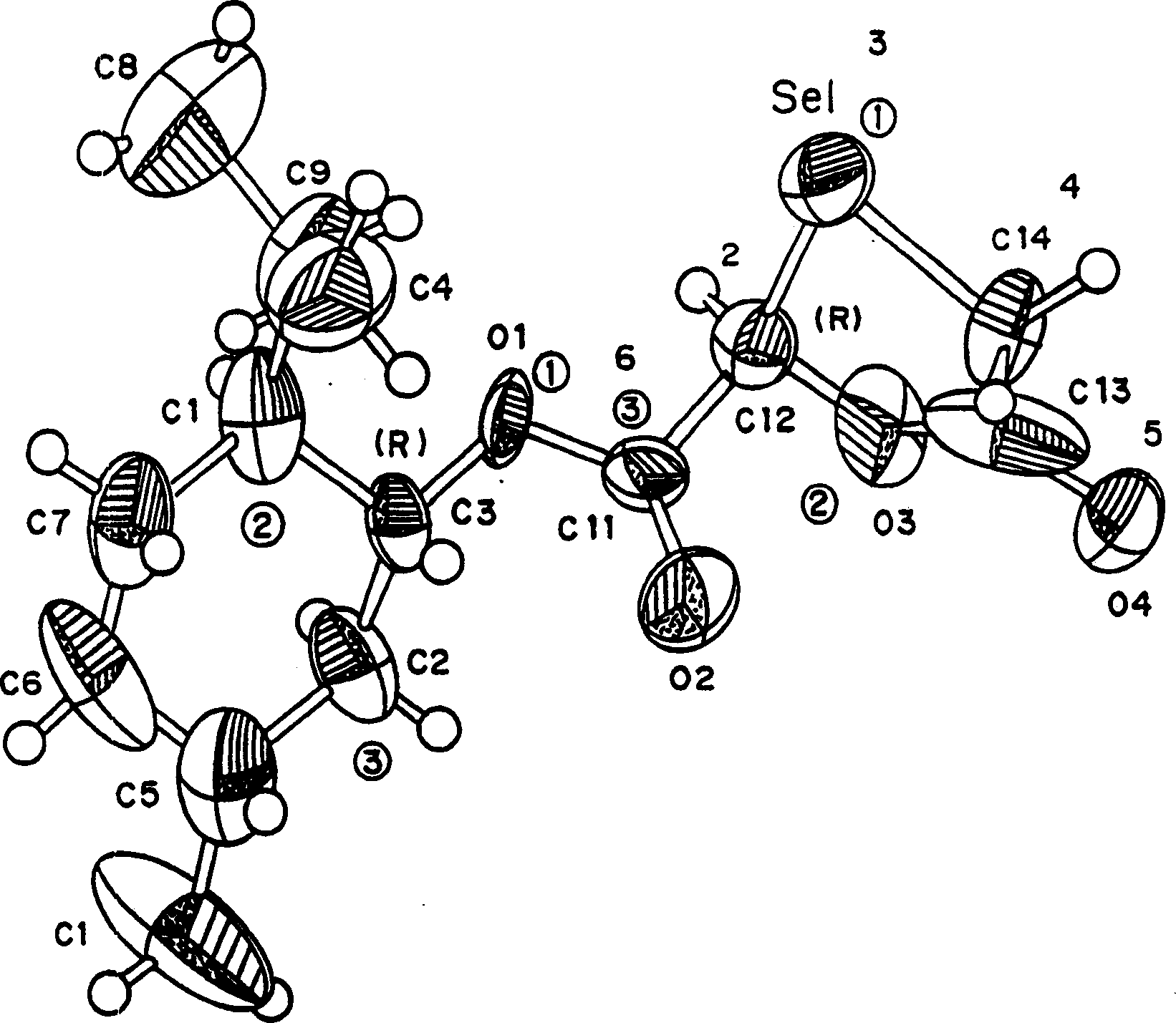
[0074] Hitherto, 1,3-oxoselenolane nucleosides have not been produced due to the difficulty in synthesizing the ring structure of 1,3-oxoselenolane. A method of producing this ring is provided herein. Figure 1 shows an embodiment of the method. Also provided herein are methods of producing isolated β-D (ie, 2S,5R) and β-L 1,3-oxoselenane (ie, 2R,5S) nucleosides. Figure 2 shows an embodiment of the method. Figure 1 also provides the numbering of the compounds used in the following examples.
Embodiment 1
[0075] Example 1 Preparation of 1,3-oxoselenolane
[0076] Application of Kirby's method to the preparation of selenocyanates gave very high yields. In the first step, ethyl bromoacetate (BrCH 2 CO 2 Et) reacts with potassium selenocyanate to form selenocyanate 2.
[0077] For the synthesis of lactone 5, an initial attempt was made to use NaBH 4 Reduction of selenocyanate 2 followed by hydrolysis of the resulting ester with NaOH yields selenolacetic acid, which can be used in the structure of the oxyselenolane system 5. However, during acidification to pH 2 with HCl, selenolacetic acid was degraded. It has been reported that selenol is easily oxidized by oxygen in air to form a stable dimer, which can be 3 PO 2 And reduced to selenol. It has been found that the reduction of bis(selenoacetic acid) to selenol and the cyclization reaction can be carried out in one reaction vessel without isolation of intermediate products. Therefore, 1 and KSeCN were refluxed in ethanol f...
Embodiment 2
[0081] Example 2 Resolution of 2-hydroxymethyl-4-(n-5'-cytosine-1'-yl)-1,3-oxyselenolane and 2-hydroxymethyl-4-( β-D and β-L enantiomers of n-5′-fluorocytosine-1′-yl)-1,3-oxoselenolane
[0082]2-Hydroxymethyl-4-(N-5′-cytosine-1′-yl)-1,3-oxoselenane and 2-hydroxymethyl-4-(N-5 '-fluorocytosine-1'-yl)-1,3-oxyselenolane. The compound (racemic, about 2 mg) was dissolved in a minimal amount (about 400 [mu]l) of methanol (HPLC grade). The conditions used for resolution are as follows: Waters HPLC system; column: Chiralpak AS 4.6×250mm; mobile phase: 2-propanol, flow rate: 0.80 ml / min; detector: UV-260nm; injection gas: helium; injection speed: 25 ml / min / solvent tank; injection volume: 20μl solution each time; retention time: (-)-(2S,5R)-β-L-2′,3′-dideoxy-3′-seleno-cytosine nucleus Glycoside, 5.50 minutes; (+)-(2R,5S)-β-D-2′,3′-dideoxy-3′-seleno-cytidine, 6.92 minutes; (-)-(2S,5R )-β-L-2′,3′-dideoxy-5-fluoro-3′-seleno-cytidine, 5.97 minutes; (+)-(2R,5S)-β-D-2′, 3'-dideoxy-5-fluor...
PUM
| Property | Measurement | Unit |
|---|---|---|
| optical purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com