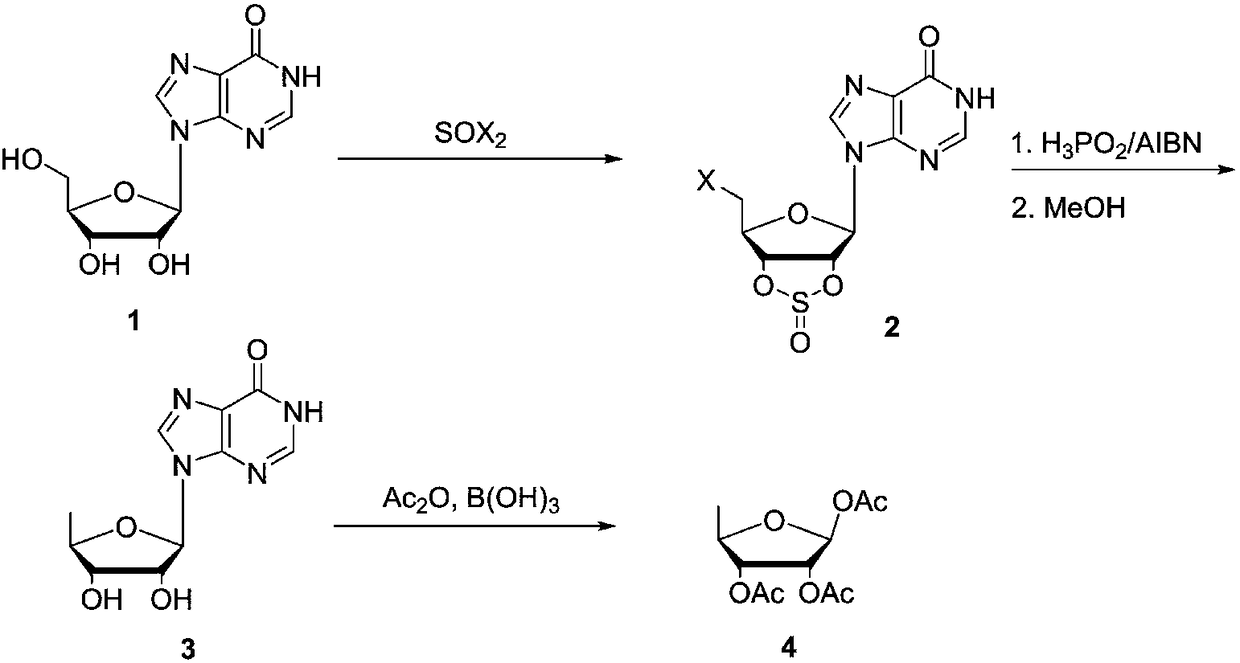
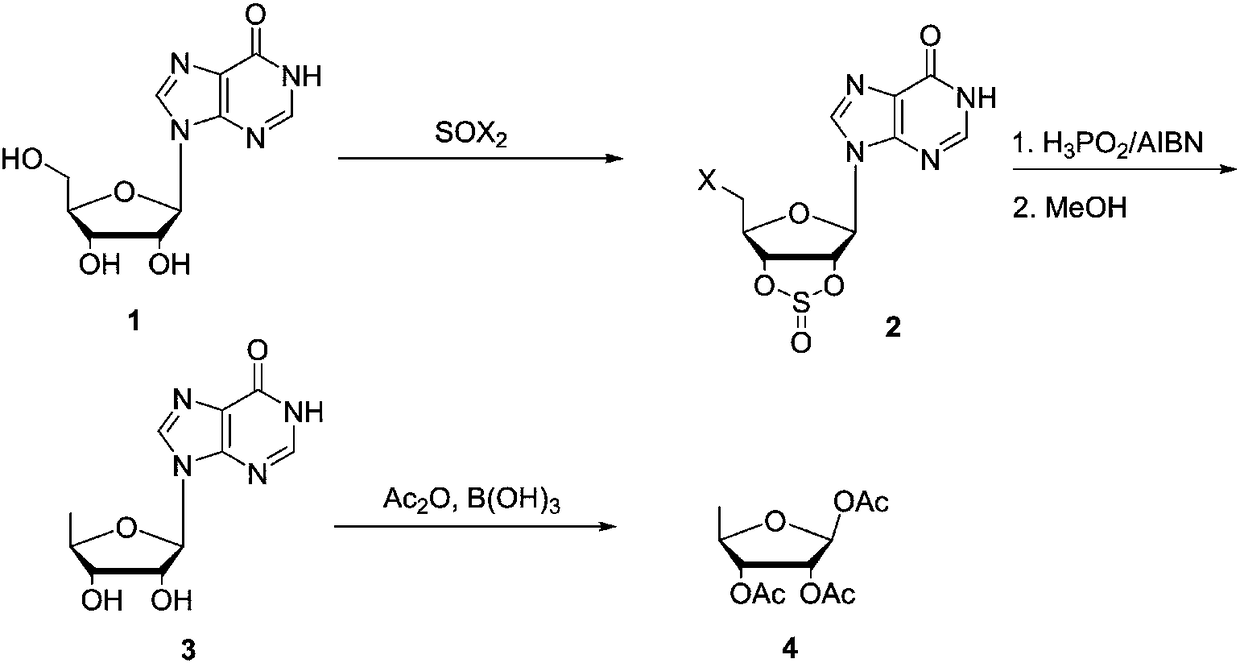
Preparing method of 1,2,3-tris-O-acetyl-5-deoxy-beta-D-ribose
An acetyl and ribose technology, applied in the preparation of sugar derivatives, chemical instruments and methods, sugar derivatives, etc., can solve the problems of difficult purification, low total yield, obvious potential safety hazards, etc., and achieve easy industrial production and reaction conditions. Mild and promising effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Preparation of 5'-chloro-2',3'-sulfoxide ester-inosine (2)
[0025] In a 500mL three-necked flask with a stirrer and a thermometer, add inosine (80g, 0.3mol) and 350mL of acetonitrile, cool down to 0°C, slowly add thionyl chloride (107g, 0.9mol) dropwise and then heat up to reflux After reacting for 5 hours, the solvent of the reaction solution was concentrated and recovered, the concentrated solution was added with ethyl acetate and stirred to precipitate a solid, suction filtered, the filter cake was washed with ethyl acetate, and dried to obtain 73g of product, yield: 73.5%.
[0026] Preparation of 5'-deoxyinosine (3)
[0027] In a 500mL three-necked flask with a stirrer and a thermometer, add 2 (50g, 0.15mol), triethylamine (45.5g, 0.45mol), hypophosphorous acid (50% aqueous solution) (59.4g, 0.45mol), AIBN (1g , 0.006mol), acetonitrile 200mL, warming up to reflux, reflux reaction for 5h, the reaction solution is concentrated and the solvent acetonitrile is recover...
Embodiment 2
[0031] Preparation of 5'-chloro-2',3'-sulfoxide ester-inosine (2)
[0032] In a 500mL three-neck flask with a stirrer and a thermometer, add inosine (80g, 0.3mol) and 350mL of acetonitrile, cool down to 0°C, slowly add thionyl chloride (95g, 0.8mol) dropwise and then heat up to reflux After reacting for 5 hours, the cooling solvent was concentrated and recovered, the concentrated solution was added with ethyl acetate and stirred to precipitate a solid, suction filtered, the filter cake was washed with ethyl acetate and dried to obtain 69g of product, yield: 69.4%.
[0033] Preparation of 5'-deoxyinosine (3)
[0034] In a 500mL three-necked flask with a stirrer and a thermometer, add 2 (50g, 0.15mol), triethylamine (30.3g, 0.3mol), hypophosphorous acid (50% aqueous solution) (39.6g, 0.3mol), AIBN (1g , 0.006mol), acetonitrile 200mL, heated to reflux, reflux reaction for 5h, the reaction solution was concentrated and solvent acetonitrile was recovered, the residue was added to ...
Embodiment 3
[0038] Preparation of 5'-bromo-2',3'-sulfoxide ester-inosine (2)
[0039] In a 500mL three-necked flask with a stirrer and a thermometer, add inosine (80g, 0.3mol) and 350mL of acetonitrile, cool down to 0°C, slowly add dibromosulfoxide (187g, 0.9mol) dropwise and then raise the temperature to reflux After reacting for 5 hours, the solvent was concentrated and recovered, the concentrated solution was added with ethyl acetate and stirred to precipitate a solid, suction filtered, the filter cake was washed with ethyl acetate and dried to obtain 80 g of product, yield: 72%. Preparation of 5'-deoxyinosine (3)
[0040]In a 500mL three-necked flask with a stirrer and a thermometer, add 2 (50g, 0.15mol), triethylamine (45.5g, 0.45mol), hypophosphorous acid (50% aqueous solution) (59.4g, 0.45mol), AIBN (1g , 0.006mol), acetonitrile 200mL, warming up to reflux, reflux reaction for 5h, the reaction solution is concentrated and the solvent acetonitrile is recovered, the residue is added...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com