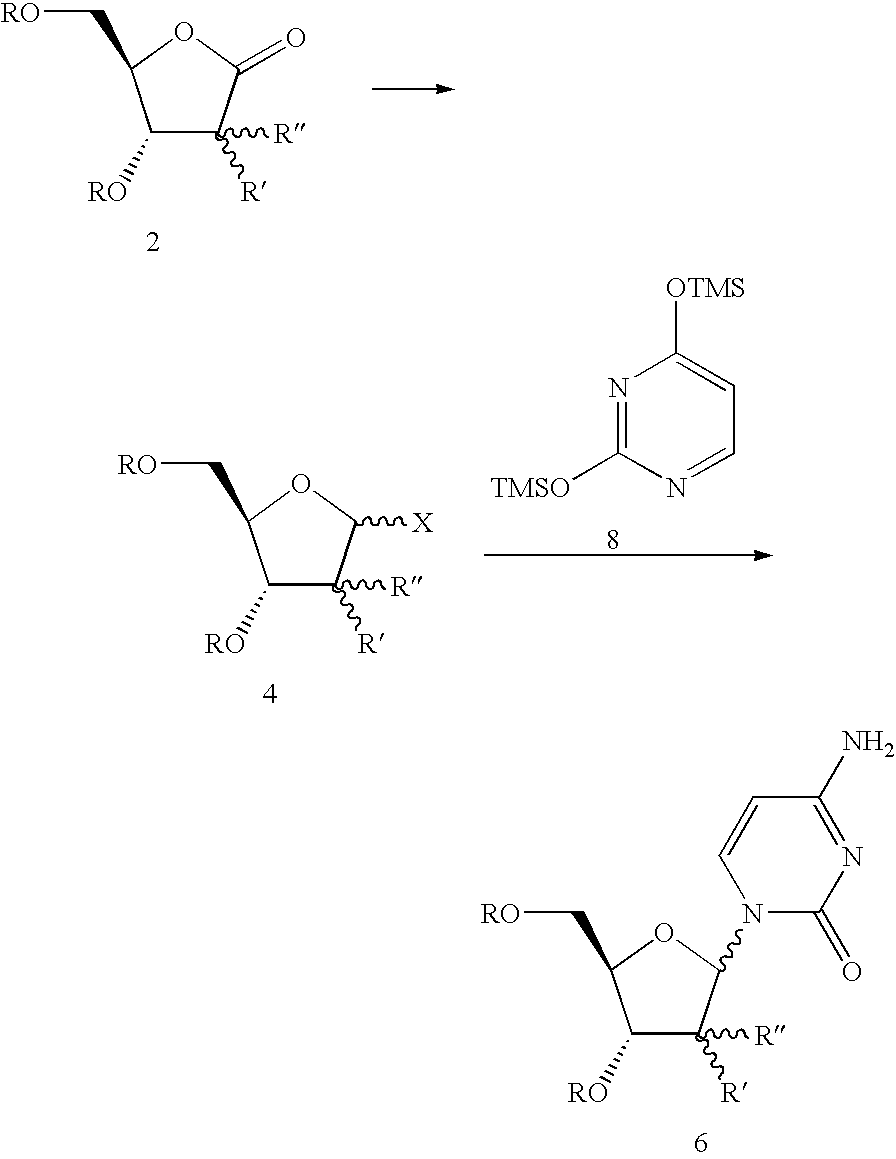
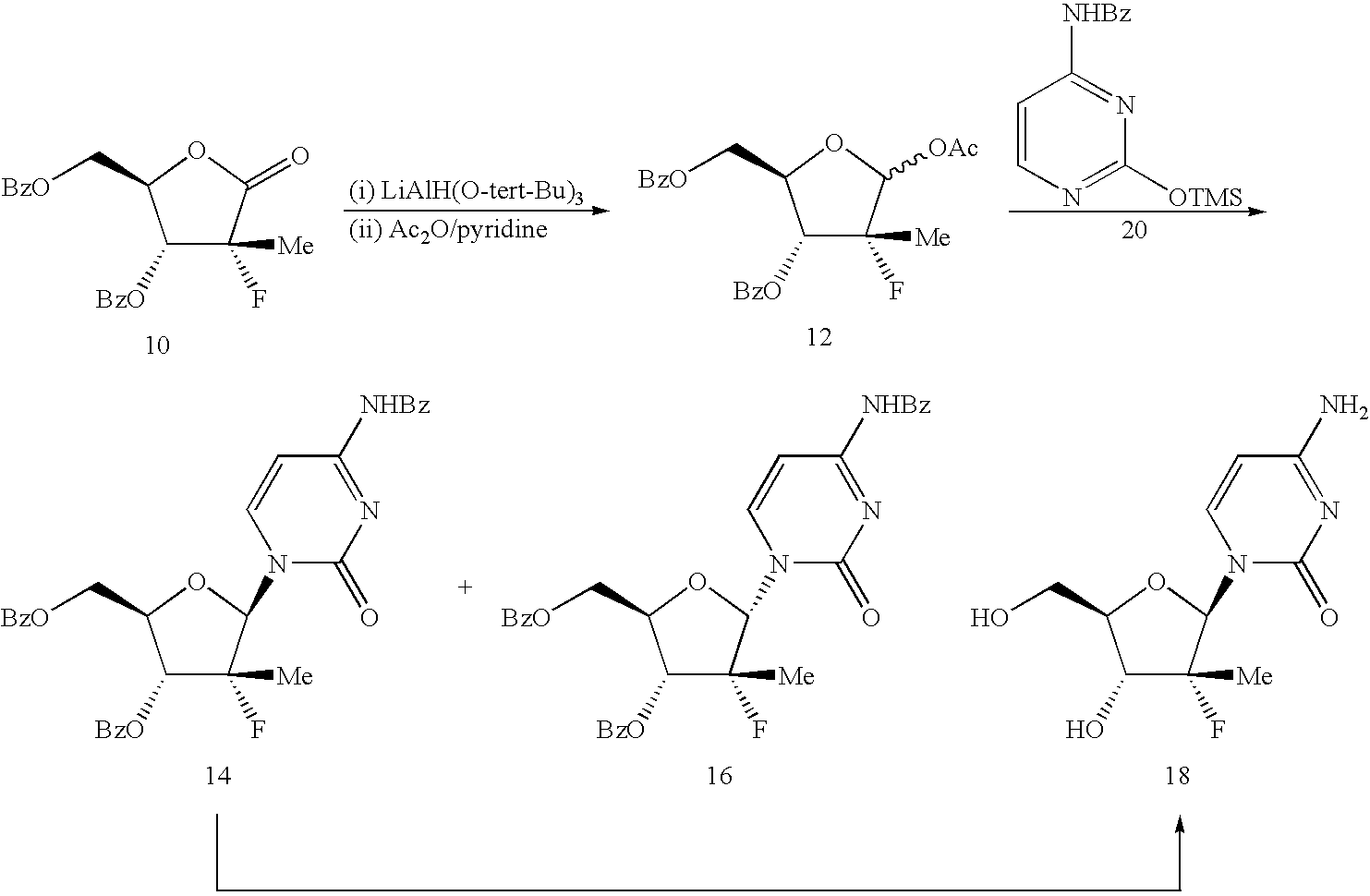
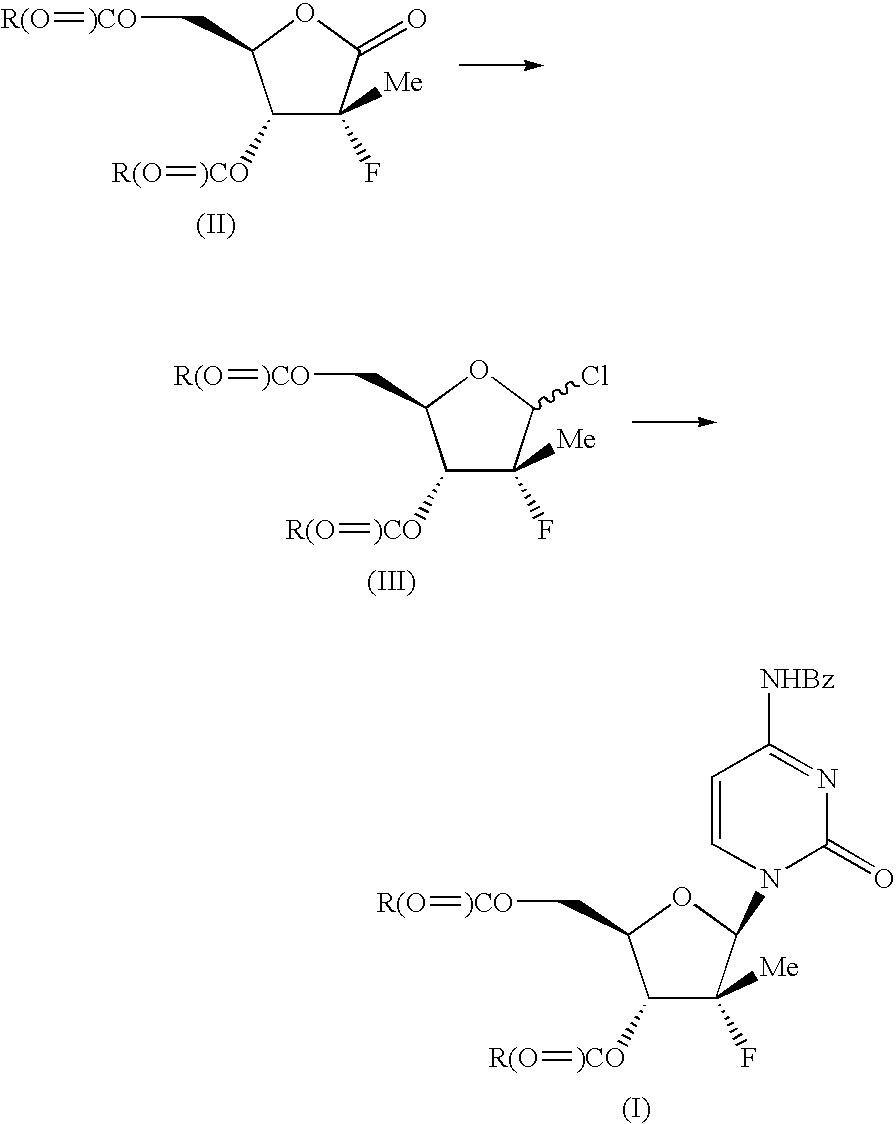
Preparation of nucleosides ribofuranosyl pyrimidines
a technology of ribofuranosyl pyrimidine and nucleosides, which is applied in the field of preparation of nucleosides ribofuranosyl pyrimidines, to achieve the effect of efficient and scalable process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Benefits of technology
Problems solved by technology
Method used
Image
Examples
example 1
Benzoic acid 3-benzoyloxy-5-(4-benzoylamino-2-oxo-2H-pyrimidin-1-yl)-4-fluoro-4-methyl-tetrahydro-furan-2-ylmethyl ester (14)
[0042]
[0043]Trifluoroethanol (4.08 kg) is added slowly to a cold solution (−15° C.) of RED-AL® solution (12.53 kg) and toluene (21.3 kg) while maintaining the reaction temperature at or below −10° C. After warming up to RT (ca. 20° C.), the modified RED-AL reagent mixture (30.1 kg out of the 37.6 kg prepared) is added slowly to a pre-cooled solution (−15° C.) of fluorolactone dibenzoate 10 (10 kg) in DCM (94.7 kg) while maintaining reaction temperature at or below −10° C. After reduction of the lactone (monitored by in-process HPLC), a catalytic amount of tetrabutylammonium bromide (90 g) is added to the reaction mixture. Sulfiiryl chloride (11.86 kg) is then added while maintaining reaction temperature at or below 0° C. The reaction mixture is then heated to 40° C. until formation of the chloride is complete (ca. 4 h) or warmed to RT (20-25° C.) and stirred o...
example 2
4-Amino-1-(3-fluoro-4-hydroxy-5-hydroxymethyl-3-methyl-tetrahydro-furan-2-yl)-1H-pyrimidin-2-one (18)
[0045]
[0046]A slurry of 14 (14.7 kg) in MeOH (92.6 kg) is treated with catalytic amounts of methanolic sodium methoxide (0.275 kg). The reaction mixture is heated to ca. 50° C. and aged (ca. 1 h) until the hydrolysis is complete. The reaction mixture is quenched by addition of isobutyric acid (0.115 kg). The resulting solution is concentrated under moderate vacuum and then residual solvents are replaced with IPA (80 kg). The batch is distilled to a volume of ca. 50 L. The resulting slurry is heated to ca. 80° C. and then cooled slowly to ca. 5° C. and aged (ca. 2 h). The precipitated product is isolated by filtration, washed with IPA (16.8 kg) and dried in an oven at 70° C. in vacuo to afford 6.26 kg (88.9%) of 18 which assayed at 99.43% pure.
example 3
(2S,3R)-3-[(4R)-2,2-dimethyl-[1,3]dioxolan-4-yl]-2,3-dihydroxy-2-methyl-propionic acid ethyl ester (24)
[0047]
[0048]A suspension of 22 (10 kg, CAS Reg. No. 81997-76-4), ethylene glycol (11.6 kg), solid NaHCO3 (11.8 kg) and acetone (150 L) is cooled to ca.-15° C. A solution of 36% aqueous NaMnO4 (19.5 kg) is charged slowly (over 4 h) to the suspension maintaining reaction temperature at or below −10° C. After stirring for 0.5 h at −10° C., an aliquot of the reaction mixture (ca. 5 mL) is quenched with 25% aqueous sodium bisulfite (ca. 15 mL). A portion of resulting slurry is filtered and submitted for GC analysis to check the progress of the reaction. When the reaction is complete, the reaction mixture is quenched by slow addition (over 40 min) of cooled (ca. 0° C.) 25% aqueous NaHSO3 (60 L). The temperature of the reaction mixture is allowed to reach 4° C. during the quench. CELITE® (ca. 2.5 kg) is then slurried in acetone (8 kg) and added to the dark brown reaction mixture. The resu...
PUM
| Property | Measurement | Unit |
|---|---|---|
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
| temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com